If you strip away the complexities of life—the beating hearts, crashing stars, roaring oceans, and silent thoughts—you’re left with something startlingly simple. Beneath it all, everything is made from atoms. The chair beneath you, the air around you, the screen you’re reading this on. Even you. We are, in the most literal and profound sense, made of stardust—collections of atoms forged in ancient cosmic furnaces, assembled into molecules that built cells, organs, and minds.
Atoms are the building blocks of matter, the alphabet from which the story of the universe is written. But even atoms are only part of the story. They organize themselves into elements—distinct types of atoms that define the chemical identity of everything in existence. Together, atoms and elements form the scaffolding of reality itself.
This is the tale of how these building blocks came to be understood, how they shape our world, and how their mysterious dance continues to reveal the hidden poetry of the cosmos.
The Ancient Dream of What Things Are Made Of
Long before particle accelerators and quantum equations, human beings gazed into the world and asked a question both childlike and profound: What is this made of?
The first answers were stories. Myths of fire and water, earth and air, danced across cultures. In ancient Greece, philosophers like Empedocles believed all matter stemmed from four elements—earth, air, fire, and water—held together by love and torn apart by strife. It was not science as we know it, but it was a beginning.
Democritus, around 400 BCE, offered a radical idea: that all matter was made of tiny, indivisible units called atomos—“uncuttable.” These invisible particles, he said, came in different shapes and sizes, bumping and swirling in an infinite void. His idea was largely ignored, overshadowed by the more spiritual notions of Plato and Aristotle.
And so, for nearly two thousand years, the atom slept in silence.
Alchemy’s Fire and the Dawn of Chemistry
While the classical world speculated, another tradition took root—alchemy. Part science, part mysticism, alchemy sought to transform matter. Its most famous goal, turning lead into gold, was driven by both greed and the belief that matter could be perfected.
In their strange laboratories, alchemists discovered acids, developed glassware, and refined metals. Though they never succeeded in creating gold from base metals, they laid the groundwork for modern chemistry. The language of elements began to emerge not through theory, but through practice.
One alchemist stood apart: Jabir ibn Hayyan, a Persian scholar of the 8th century. In his writings, we see the stirrings of method—a careful observation of substances, the idea of reproducible experiments. Though still steeped in mysticism, Jabir’s work helped nudge the ancient dream toward something more scientific.
The Elemental Revolution
It wasn’t until the 17th and 18th centuries that the true nature of matter began to be revealed. As science blossomed in Europe, chemists started cataloging the behaviors of different substances. They noticed regularities: certain materials could not be broken down further. These were the elements—substances made of only one type of atom.
In 1789, French chemist Antoine Lavoisier published a list of 33 known elements. He defined an element as a substance that could not be broken down into anything simpler by chemical means. It was a moment of clarity—a crisp departure from alchemical fantasy.
With Lavoisier’s work, chemistry became a true science. He showed that matter was conserved in reactions, and that gases like oxygen were real, measurable things. He helped cut through centuries of mysticism, even losing his life during the French Revolution for his efforts. “The Republic has no need of scientists,” the judge said before ordering his execution.
But the revolution he sparked could not be beheaded. It had only begun.
The Secret Code of the Elements
As more elements were discovered—iron, copper, sulfur, carbon, mercury, tin—the question arose: could there be a pattern?
In the 1860s, a quiet, methodical Russian chemist named Dmitri Mendeleev arranged the known elements by atomic weight and noticed something extraordinary. Certain properties recurred in a regular pattern. When he left gaps in his table, he boldly predicted that unknown elements would fill them. And they did. The Periodic Table was born—not merely a list, but a map of the atomic world.
Mendeleev’s brilliance lay not only in his organization, but in his insight: that elements are not arbitrary. They follow rules written into the very nature of atoms. But no one yet knew what atoms truly were.
That mystery would soon be shattered.
Peering into the Atom
At the dawn of the 20th century, science plunged into the heart of the atom—and found it was not indivisible after all.
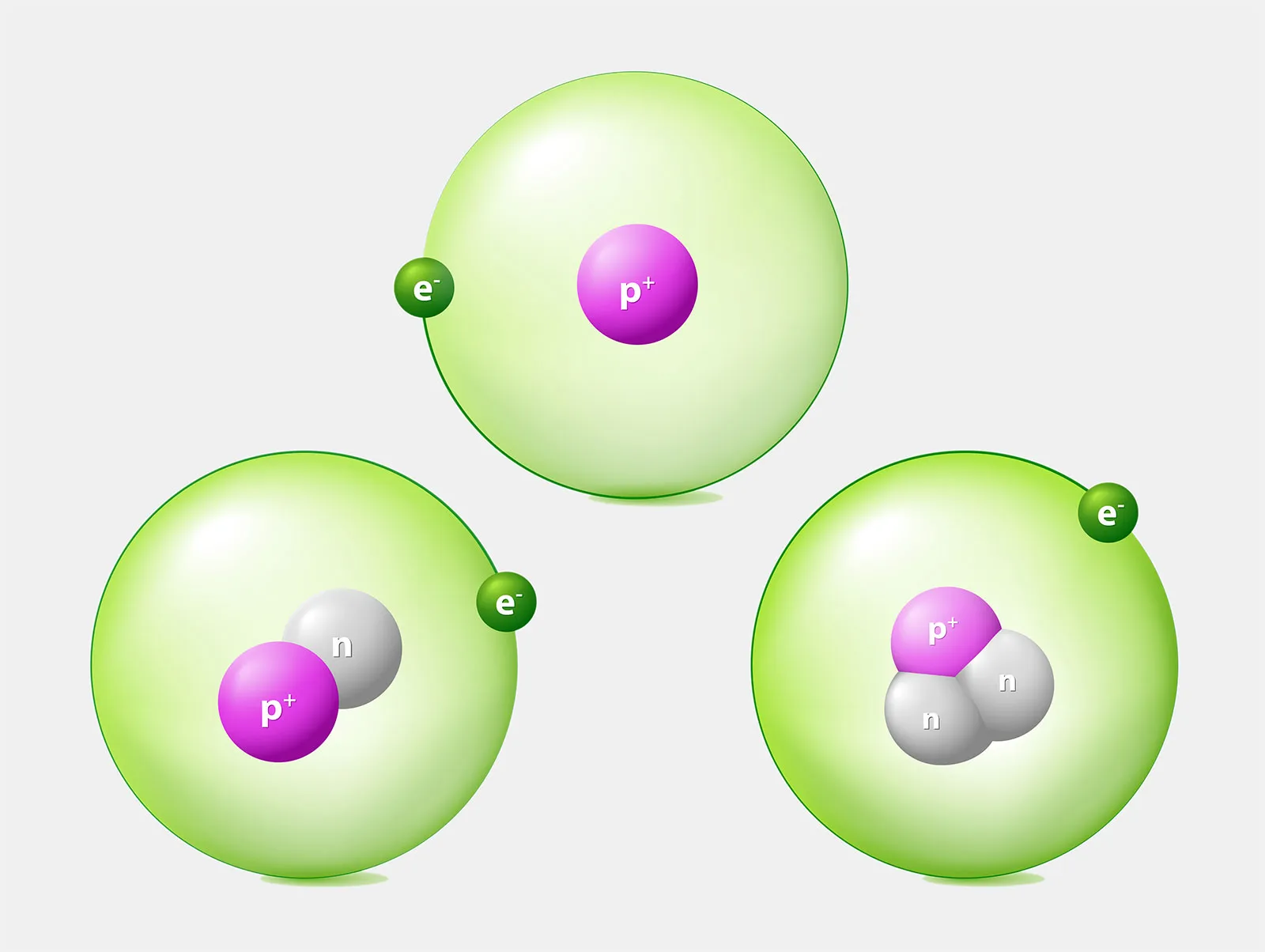
In 1897, J.J. Thomson discovered the electron, a tiny, negatively charged particle swirling inside the atom. This was astonishing. Atoms, once thought solid and unbreakable, had parts.
Soon after, Ernest Rutherford’s gold foil experiment revealed that atoms consist of a dense, positively charged nucleus surrounded by electrons. Most of the atom, to everyone’s shock, was empty space.
The nucleus itself contained protons—heavy, positively charged particles. Later, in 1932, James Chadwick discovered the neutron, an equally heavy but neutral partner to the proton. These three—the proton, neutron, and electron—became the holy trinity of atomic structure.
Each element, it turned out, was defined by the number of protons in its nucleus. Hydrogen has one. Helium has two. Carbon has six. Uranium has ninety-two. The number of protons, called the atomic number, gives an element its identity. Add or remove protons, and you get a new element.
Neutrons and electrons, meanwhile, shape the atom’s mass and behavior. Isotopes, for instance, are versions of the same element with different numbers of neutrons. They can be stable, like carbon-12, or unstable and radioactive, like carbon-14.
And electrons? They dance in shells, orbitals, and clouds, forming the outer skin of atoms. Their arrangements determine how atoms bond, react, and form molecules. They are the matchmakers of chemistry.
Where Atoms Are Born
With the atom’s structure revealed, the next great question loomed: where did atoms come from?
For that, we must look to the stars.
In the earliest moments of the universe—just minutes after the Big Bang—only the lightest elements existed. Hydrogen, helium, and a touch of lithium emerged as the universe cooled. They were born in a cosmic fireball, scattered across a dark, expanding void.
Heavier elements had to wait.
Inside stars, immense pressure and heat force atoms to collide and fuse, forming heavier elements. Hydrogen becomes helium. Helium becomes carbon, oxygen, and so on—up to iron. This process, called nuclear fusion, powers the stars and creates the elemental building blocks of life.
But iron is a dead end. It cannot release energy by fusion. So how do we get gold, uranium, or iodine?
When massive stars die, they explode in supernovae—cataclysmic bursts that reach temperatures high enough to forge the heaviest elements in moments. Some may even form in the collision of neutron stars, an event so violent it literally shakes the fabric of space-time.
And so, the elements that make up your blood, your bones, your breath, were born in stars. You are, quite literally, a way for the universe to know itself.
The Language of Chemistry
With atoms and elements understood, chemistry became a language—one spoken in bonds, reactions, and transformations.
When atoms bond, they share or transfer electrons to achieve stability. Covalent bonds—like those in water or DNA—are formed when atoms share electrons. Ionic bonds—like those in salt—occur when electrons are transferred.
These bonds form molecules: water (H₂O), carbon dioxide (CO₂), glucose (C₆H₁₂O₆), and millions more. Molecules interact in reactions, building and breaking structures, releasing and absorbing energy. Chemistry is not just about what things are—it’s about how they change.
In biology, chemistry becomes life. DNA is a molecule. Proteins are molecules. Nerve impulses, digestion, thought itself—all are chemical processes unfolding in real time.
In industry, chemistry becomes transformation. Plastics, medicines, fuels, fertilizers, electronics—each is a child of the chemist’s art, born from the manipulation of atoms and elements.
In art, chemistry becomes color. Pigments, glazes, fireworks—all derive from elements arranged to produce stunning visual effects.
The periodic table, once a simple chart, now glows with meaning. Each element is a note in the symphony of matter. Each atom, a player in the great play of existence.
Into the Quantum Realm
Just as science once shattered the indivisible atom, it later shattered the certainty of its behavior.
Quantum mechanics, developed in the early 20th century, revealed that particles like electrons do not move in tidy orbits but in clouds of probability. They can behave like waves or particles, exist in multiple states, and influence each other at a distance—a phenomenon called entanglement.
In this realm, certainty gives way to probability. Electrons don’t have fixed locations, only likely ones. Atoms don’t behave like tiny solar systems, but like fuzzy puzzles.
Quantum theory doesn’t make chemistry impossible. It makes it deeper. The periodic table reflects quantum rules—electron shells, orbital shapes, energy levels. The behavior of elements is encoded in the strange, beautiful logic of quantum physics.
It’s a world where intuition fails, but the math never lies.
Creating the Unnatural: Man-Made Elements
Not all elements are born in stars.
In laboratories, scientists have learned to create new elements by smashing atoms together at high speeds. These synthetic elements live at the bottom of the periodic table—heavy, unstable, and exotic. Names like Californium, Einsteinium, and Oganesson tell of human achievement as much as atomic structure.
These elements often last only fractions of a second before decaying, but they prove a vital truth: we are no longer just discovering nature’s elements. We are adding to them.
Each synthetic atom is a monument to human ingenuity, a fragile whisper of matter shaped by particle accelerators and theoretical predictions.
Some hope to reach the “island of stability”—a hypothetical region of superheavy elements that might be unusually long-lived. If such elements exist, they could open up entirely new realms of chemistry, material science, or even quantum computing.
Atoms, Life, and the Edge of Understanding
In the end, atoms and elements are not just components of things. They are the storytellers of existence.
Carbon, versatile and bonding with ease, forms the backbone of life. Oxygen fuels the fires of metabolism. Iron carries our blood. Phosphorus encodes our genes. Calcium shapes our bones.
We are not merely made of atoms—we are animated by them, organized by them, sustained by their restless energy.
But the story is not complete.
Dark matter and dark energy hint at deeper structures beyond atoms. String theory suggests that particles may be vibrations of tiny loops. Quantum field theory paints particles as ripples in invisible fields.
And yet, for all our complexity, we return to simplicity. A hydrogen atom—one proton, one electron—is still the most abundant structure in the universe. From such humble particles comes everything: stars, oceans, poets, and possibility.
The Ongoing Discovery
Even now, in the 21st century, we continue to peel back layers of atomic truth.
New technologies let us manipulate atoms one by one. Quantum computers aim to harness their strangeness. Nanotechnology builds materials from the bottom up. Fusion power, the dream of bottled stars, edges closer to reality.
The periodic table has not finished growing. Elements 119 and 120 remain targets of synthesis. Each discovery extends the boundary of the known—and reveals new mysteries beyond.
Science is no longer just about what atoms are, but about what they can do—how we can design, engineer, and even dream with them.
Atoms, once hidden and hypothetical, are now tools of creation. The ancient question—what is everything made of?—has been answered in part. But the greater question—what can we become with this knowledge?—remains beautifully open.