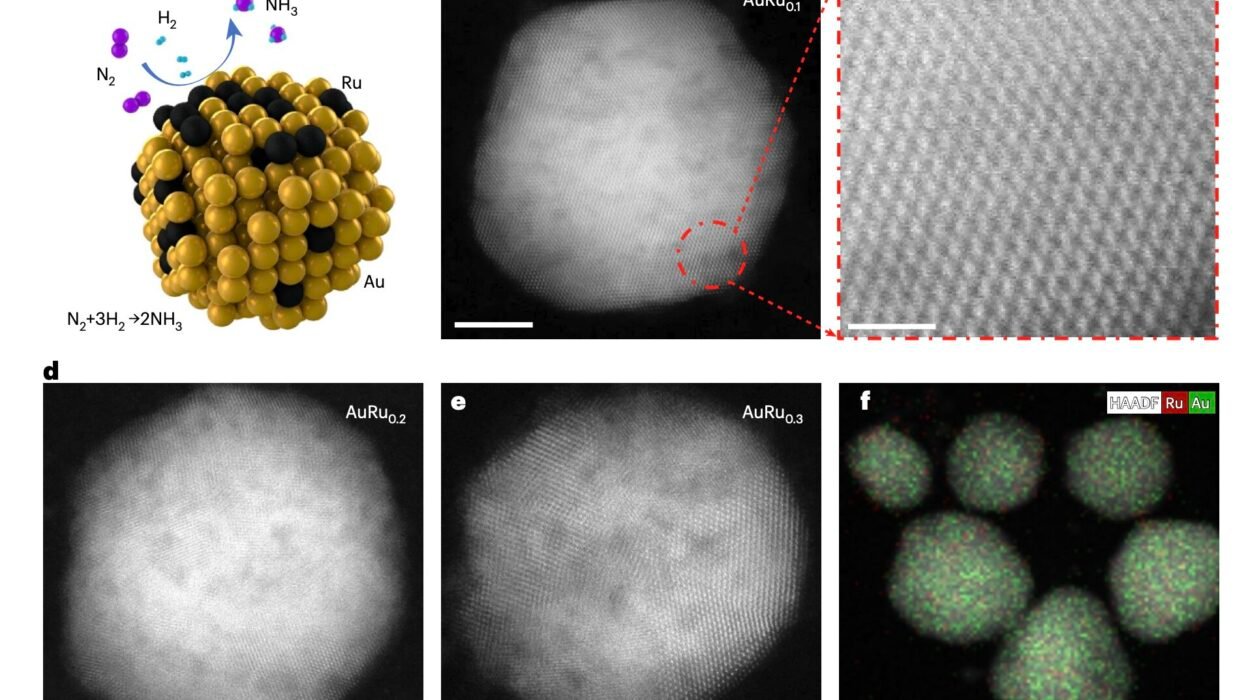
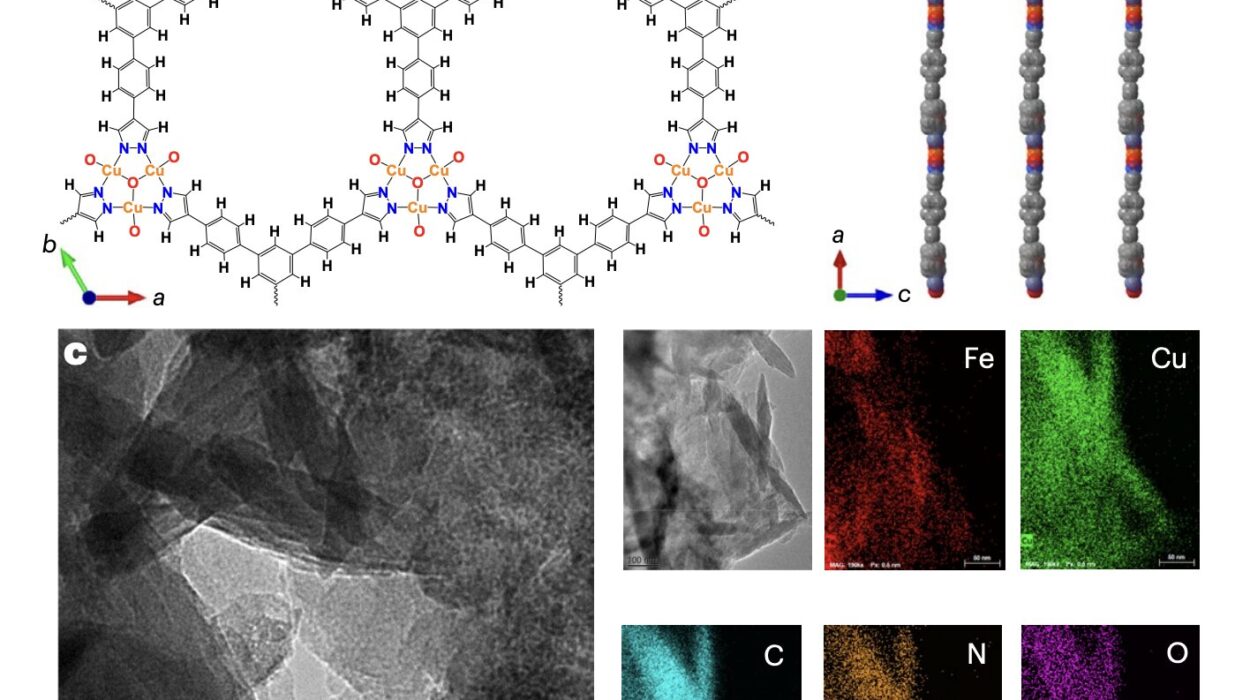
If you could zoom in to the tiniest scales of matter, beyond cells and molecules, you would discover a world ruled by giants—long, flexible chains of repeating units called polymers. Though invisible to the naked eye, these molecular titans quietly weave the fabric of modern life and nature itself. Every time you sip water from a plastic bottle, wear a raincoat, or simply blink your eyes, polymers are at work. They make up synthetic materials like nylon and rubber, but also the most fundamental components of life—DNA, proteins, and carbohydrates.
Polymers are not merely materials; they are architects of function. They determine how your smartphone casing resists scratches, how your blood vessels stretch and contract, how spider silk defies its size to become stronger than steel. The story of polymers is one of ingenuity and discovery, of molecules that connect human invention to biological marvels. To understand their power is to see the hidden threads binding technology and life into one vast molecular tapestry.
What Exactly Are Polymers?
At their heart, polymers are deceptively simple. They are large molecules composed of repeating smaller units known as monomers, linked together like beads on a string. These chains can stretch to enormous lengths, containing thousands or even millions of monomers. Yet the beauty of polymers lies not only in their size but in how these chains can fold, coil, and entangle, creating properties far beyond those of individual monomers.
Imagine spaghetti on a plate. Each strand represents a polymer chain. If the spaghetti were short and rigid, it wouldn’t twirl easily on a fork. But long, flexible strands can knot, tangle, and stretch—just like polymer chains. This ability to move and twist gives polymers their remarkable versatility.
Nature has been crafting polymers for billions of years. DNA, the molecule that carries genetic instructions for life, is a natural polymer made of nucleotide monomers. Proteins, which build and regulate our bodies, are polymers of amino acids. Even cellulose, the material that gives plants their structure, is a polymer of glucose. Long before humans learned to make plastics, nature had already mastered polymer science.
The Birth of Synthetic Polymers
Humanity’s journey into synthetic polymers began not in advanced laboratories, but in kitchens, workshops, and curious experiments. In the 19th century, as industrialization swept the world, inventors sought materials that could mimic or surpass natural substances like ivory and silk. The search led to a series of accidental discoveries that would forever change material science.
One of the first breakthroughs came in 1839, when Charles Goodyear discovered vulcanization—a process that made natural rubber more durable by heating it with sulfur. This laid the foundation for tires, waterproof clothing, and countless other products.
Later, in 1907, Leo Baekeland introduced Bakelite, the first fully synthetic plastic. It was a hard, moldable material resistant to heat and electricity, quickly finding its way into telephones, radios, and kitchenware. Society had glimpsed the potential of crafting entirely new materials by manipulating polymer chains.
From there, innovation exploded. Nylon, discovered in 1935, offered a synthetic alternative to silk. Polyethylene, the polymer behind plastic bags, emerged soon after. These materials were lightweight, inexpensive, and versatile, triggering a revolution that transformed industries from packaging to aerospace.
The Science Behind Polymer Properties
What makes polymers so extraordinary is the range of properties they can exhibit. A polymer can be as soft as a rubber band or as rigid as a bulletproof vest. It can conduct electricity like a metal or insulate against it like glass. The secret lies in the chemistry of the monomers, the length of the chains, and how those chains interact with each other.
When polymer chains are loosely entangled, they behave like liquids, flowing easily. When cross-linked—connected by chemical bonds—they form tough, elastic solids. Some polymers align into crystalline regions, making them strong and stiff, while others remain amorphous, giving them flexibility.
This tunability is what allows scientists to design polymers for specific purposes. Kevlar, for example, is made of rigid polymer chains aligned in parallel, producing fibers with exceptional tensile strength used in body armor. Teflon’s polymer chains create a slippery, non-stick surface perfect for cookware. Hydrogels, soft water-swollen polymers, are engineered to mimic biological tissues for medical applications.
The chemistry of polymers is not just about structure but also about dynamics. These chains can stretch, snap back, slide past one another, or even self-heal. They can respond to temperature, pH, light, or electric fields, opening the door to “smart materials” that adapt to their environment.
Polymers in Nature: Life’s Molecular Framework
Long before humans synthesized plastics, nature evolved its own polymeric machinery to sustain life. DNA, perhaps the most iconic natural polymer, carries the instructions that define every organism. Its double-helix structure, with repeating nucleotide units, not only stores information but replicates itself with astounding accuracy, ensuring continuity of life.
Proteins, another class of natural polymers, fold into intricate three-dimensional shapes that allow them to perform specific tasks. Enzymes, which speed up chemical reactions, and antibodies, which protect against disease, are all polymeric masterpieces.
Carbohydrates like starch and cellulose are polymers of sugar molecules, providing energy storage and structural support. Chitin, found in the exoskeletons of insects and crustaceans, is a natural polymer that offers both strength and flexibility. Even spider silk, renowned for its incredible strength-to-weight ratio, is a protein-based polymer spun with precision that outperforms many synthetic materials.
These natural polymers demonstrate the elegance and efficiency of molecular design honed by evolution. By studying them, scientists gain inspiration for creating synthetic polymers that emulate or enhance these biological functions.
The Plastic Revolution: Triumphs and Troubles
The 20th century saw polymers become ubiquitous as plastics reshaped every facet of human life. Lightweight, cheap, and moldable, plastics replaced glass, metal, and wood in countless applications. From food packaging to medical devices, synthetic polymers delivered convenience and innovation on an unprecedented scale.
Plastics helped democratize products once considered luxuries. Clothing made from synthetic fibers became affordable for the masses. Automotive and aerospace industries relied on polymers to reduce weight and improve fuel efficiency. In medicine, polymer-based sutures, prosthetics, and drug delivery systems saved lives.
Yet this triumph carried unintended consequences. Plastics’ durability, once a selling point, became an environmental curse. Unlike natural polymers that biodegrade, many synthetic plastics persist for centuries, accumulating in oceans and ecosystems. Microplastics now contaminate food chains, and discarded plastics clog landfills, posing a global challenge.
Scientists are racing to develop biodegradable polymers, recycle existing plastics, and design materials that break down harmlessly after use. The future of polymers must balance human needs with ecological responsibility, harnessing their power without drowning the planet in waste.
Polymers in Medicine: Healing with Molecular Chains
Beyond their role in industry and everyday products, polymers have revolutionized medicine. Biocompatible polymers are used to create sutures that dissolve inside the body, implants that integrate with bone, and stents that hold arteries open.
Drug delivery systems employ polymers to release medication slowly and precisely, targeting specific tissues while minimizing side effects. Hydrogels, which can hold large amounts of water, are engineered to mimic human tissues, enabling wound dressings that promote healing and artificial cartilage for joint repair.
Even cutting-edge therapies like tissue engineering and regenerative medicine rely on polymers as scaffolds where cells can grow, forming new organs or repairing damaged ones. Polymer science is not only saving lives but redefining the boundaries of what medicine can achieve.
The DNA Connection: Polymers as Information Carriers
Among all polymers, DNA stands out as the most extraordinary. This biological macromolecule is a polymer of four types of nucleotides, strung together in sequences that encode genetic instructions. The length of DNA polymers is staggering—if uncoiled, the DNA in a single human cell would stretch over two meters, yet it fits within a microscopic nucleus.
The polymeric nature of DNA is essential to its function. Its ability to replicate, mutate, and recombine underlies evolution and diversity of life. Advances in polymer chemistry and biotechnology have enabled scientists to manipulate DNA, leading to breakthroughs in genetic engineering, forensic science, and medicine.
RNA, a close relative of DNA, is also a polymer that plays crucial roles in translating genetic information into proteins and regulating cellular functions. Understanding these natural polymers has not only revealed the secrets of life but inspired synthetic biology, where artificial polymers mimic or enhance biological processes.
Smart Polymers and the Materials of Tomorrow
The future of polymer science is brimming with possibilities. Researchers are designing “smart polymers” that respond to stimuli like temperature, light, or pH. Imagine fabrics that adjust their breathability based on weather, coatings that heal scratches automatically, or medical implants that release drugs only when triggered by specific conditions.
Conductive polymers, which can carry electric current, are enabling flexible electronics, lightweight batteries, and wearable sensors. In nanotechnology, polymers are used to build molecular machines and targeted drug delivery systems. Biodegradable polymers promise sustainable alternatives to conventional plastics, while advanced composites made of polymer matrices combined with fibers are creating materials stronger than steel yet lighter than aluminum.
Polymers are also at the forefront of space exploration. From lightweight spacecraft components to high-performance insulating materials, they make missions safer and more efficient. As humanity reaches for Mars and beyond, polymers will be essential companions in surviving harsh extraterrestrial environments.
The Philosophy of Polymers: Connectivity and Adaptability
Beyond their practical applications, polymers offer profound metaphors for understanding complexity and resilience. Their strength comes not from rigidity but from connectivity and adaptability. Polymer chains can stretch and recover, endure stress, and self-organize into patterns that give rise to new properties.
In this way, polymers mirror life itself—flexible, interconnected, capable of evolving solutions to challenges. They remind us that power often lies not in isolated units but in bonds that link them together, creating something far greater than the sum of parts.
The Endless Frontier
From the genetic code that defines life to the plastics that shape modern civilization, polymers have always been silent yet powerful forces guiding human destiny. Their story is one of discovery and duality: of astonishing benefits and sobering consequences, of natural elegance and synthetic ingenuity.
As science advances, we stand on the edge of a new era where polymers may help solve humanity’s greatest challenges—from sustainable materials to curing diseases, from unlocking biological mysteries to exploring distant worlds.
To grasp the power of polymers is to recognize the invisible giants that hold our world together. They are the molecular storytellers that write life’s narrative and fuel human creativity. In every strand of DNA, every plastic tool, every futuristic material, polymers quietly remind us that the universe is built not only from atoms but from the bonds that unite them—long, enduring, and endlessly transformative.