In a quiet laboratory in Giessen, Germany, a sliver of chemical history was written—a moment more than 200 years in the making. Nitrogen, the most abundant gas in Earth’s atmosphere, has always been regarded as a stable, stoic element, stubbornly clinging to its familiar form: N₂, a tightly bonded pair of atoms that make up 78% of the air we breathe.
But now, scientists from Justus Liebig University have achieved what generations of chemists thought nearly impossible—they’ve synthesized neutral hexanitrogen (N₆), a molecule composed of six nitrogen atoms in a linear chain. It’s the first time since the 18th century that a new neutral allotrope—a structurally distinct molecular form—of nitrogen has been experimentally created and isolated.
Published in the journal Nature, this milestone doesn’t just expand the periodic table’s family of known molecular structures. It also opens up bold possibilities in the quest for high-density, clean energy storage—using nothing more than the air around us.
A Nitrogen Star Is Born
The breakthrough came not from a large collider or deep-space probe, but from a deceptively simple tabletop reaction. Researchers delicately spread a thin layer of a compound known as silver azide (AgN₃)—a notoriously sensitive and explosive solid—onto a reaction surface. Then, under reduced pressure and at room temperature, they flowed a halogen gas such as chlorine (Cl₂) or bromine (Br₂) over the surface.
It was an elegant act of chemical choreography. As the halogen gases reacted with the silver azide, a complex cascade of reactions unfolded. Within moments, out of that swirl of energetic chaos, a rare molecule began to emerge: a chain of six nitrogen atoms, connected in a previously unseen arrangement. Welcome to the world, N₆.
To catch and preserve this fragile formation before it vanished, the scientists trapped it in a solid argon matrix—a kind of molecular freezer—cooled to just 10 Kelvin, a temperature colder than Pluto. There, this elusive compound could be isolated and studied in its pure form.
A Ghost Among Molecules
For decades, chemists have dreamed of building bigger molecules out of nitrogen, but every attempt at crafting polynitrogen chains had ended in disappointment—or detonation. Nitrogen’s most stable form, N₂, is held together by one of the strongest triple bonds in nature. This bond is so tight, so unyielding, that it resists most efforts to rearrange or reconfigure the element into anything larger.
Previous sightings of more complex nitrogen structures—like the azide radical (N₃) or nitrogen tetramer (N₄)—have been fleeting. Observed only through spectroscopy and never truly caught, these ghostly molecules hinted at the possibilities but remained theoretical.
Until now.
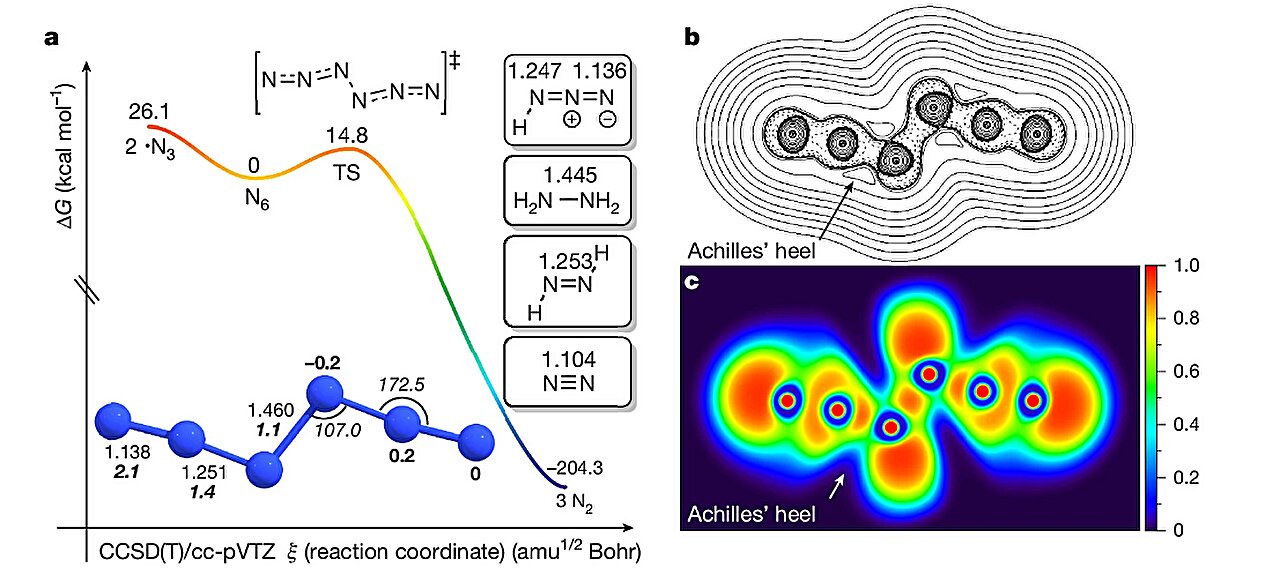
The structure of N₆ was not only synthesized but confirmed. Acyclic and linear, the molecule has C₂h symmetry—an elegant arrangement with two azide units flanking a central chain, held together by alternating double and single nitrogen bonds. A molecule of balance, tension, and stored energy.
Energy Packed in Pure Nitrogen
And what energy it holds.
Computational simulations showed that when N₆ decomposes—breaking apart and reverting back to stable N₂ gas—it releases more than twice the energy per unit mass as RDX, a powerful military-grade explosive. Even more astonishing, it delivers 2.2 times the energy of TNT.
But unlike TNT or RDX, which leave behind harmful residues and greenhouse gases, the decomposition of N₆ yields only inert nitrogen gas—clean, harmless, and abundant. In the search for future fuels that are energy-rich but carbon-free, N₆ could be a game-changer.
Its only challenge? Stability. At room temperature, N₆ has a fleeting existence: a calculated half-life of just 35.7 milliseconds. But at cryogenic temperatures—around 77 Kelvin, or the temperature at which nitrogen liquefies—it becomes remarkably stable, with a theoretical half-life of 132 years.
In other words, N₆ could be stored indefinitely in ultra-cold environments, then potentially used as a high-energy material when needed, releasing enormous power with no carbon footprint.
How the Reaction Works
The synthesis followed a two-step chemical dance. In the first act, the halogen gas reacted with silver azide to produce halogen azide (ClN₃ or BrN₃) and a byproduct, silver halide (AgCl or AgBr). Then, this newly formed halogen azide encountered another molecule of silver azide—triggering the formation of the hexanitrogen chain.
What might seem like a laboratory curiosity is actually a carefully choreographed reaction rooted in years of theoretical modeling. Chemists had long predicted the possibility of N₆ and even larger nitrogen chains like N₈ or N₁₂, but these remained theoretical flights of fancy—until now.
A Gateway to Green Energy?
Though hexanitrogen is far from being ready for commercial energy use, its implications are profound.
In a world increasingly desperate for carbon-neutral energy sources, molecules like N₆ may offer a tantalizing glimpse of what could come next. Unlike fossil fuels, which belch CO₂ into the atmosphere, decomposing N₆ would produce only nitrogen—the same gas we already inhale with every breath.
And unlike lithium-ion batteries, which rely on scarce and often environmentally destructive minerals, nitrogen is everywhere. It’s free. It’s clean. And it’s powerful.
If future materials can be developed to stabilize or harness N₆-like structures under practical conditions, we could be looking at an entirely new class of energy storage devices—lightweight, powerful, and environmentally benign.
A Scientific Frontier Reopened
Beyond its technological promise, the synthesis of N₆ is a triumph of basic science—a reminder that even the most familiar elements can surprise us.
For more than two centuries, nitrogen was seen as a closed book—simple, inert, unyielding. Now, that book has a new chapter. With hexanitrogen, nitrogen steps onto the same stage as carbon, phosphorus, and sulfur—elements known for forming multiple, stable allotropes with wildly different properties.
This opens up a new frontier in chemistry, where the rules are not just bent but rewritten.
Looking Ahead
The road to real-world applications will be long. Handling N₆ outside cryogenic labs will be risky. Engineering practical storage systems for a material so volatile will be complex. And scaling up the production safely will be a major hurdle.
But the discovery of hexanitrogen is more than a scientific first—it’s an invitation. An invitation to imagine new kinds of fuels, new paradigms for energy, and a new understanding of the elements we thought we already knew.
As humanity looks toward a cleaner, more sustainable future, it’s discoveries like this—unexpected, elegant, and born in the cold quiet of a university lab—that may light the way.
Because sometimes, the future is hidden in the heart of a molecule waiting to be discovered.
Reference: Weiyu Qian et al, Preparation of a neutral nitrogen allotrope hexanitrogen C2h-N6 , Nature (2025). DOI: 10.1038/s41586-025-09032-9