Every living cell is like a bustling city, filled with highways, factories, and power plants. At the heart of this metropolis are the mitochondria—tiny organelles that churn out adenosine triphosphate (ATP), the molecule that fuels nearly every process of life. Without them, our muscles couldn’t contract, our brains couldn’t think, and our hearts couldn’t beat.
But mitochondria are no ordinary residents of the cell. They carry a mysterious origin story that stretches back more than a billion years, to a time when life on Earth was still primitive. Long ago, an ancient archaeal cell formed an unlikely alliance with a bacterium. Instead of one consuming the other, they joined forces, each bringing unique strengths to the partnership. That ancient union became permanent, and today every mitochondrion in our bodies carries echoes of that shared ancestry.
Yet mitochondria are not completely independent. Over time, they handed most of their genes to the host cell’s nucleus. As a result, the proteins mitochondria need to function must be built outside them, then imported back in—like power plants that depend on foreign engineers to keep their machinery running. This delicate process is one of biology’s great balancing acts.
A Puzzle at the Heart of Cellular Life
For decades, scientists believed they had a fairly clear picture of how mitochondria get their proteins. The model was simple: the cell’s ribosomes—tiny molecular machines that read genetic instructions and build proteins—finish constructing an entire protein in the cytosol (the watery interior of the cell). Once complete, the protein is escorted to the mitochondrion and threaded through its membrane channels like a finished product being delivered to a factory floor.
But new research from Caltech reveals that the story is far more intricate—and far more beautiful—than the old model suggested.
Folding the Unfoldable
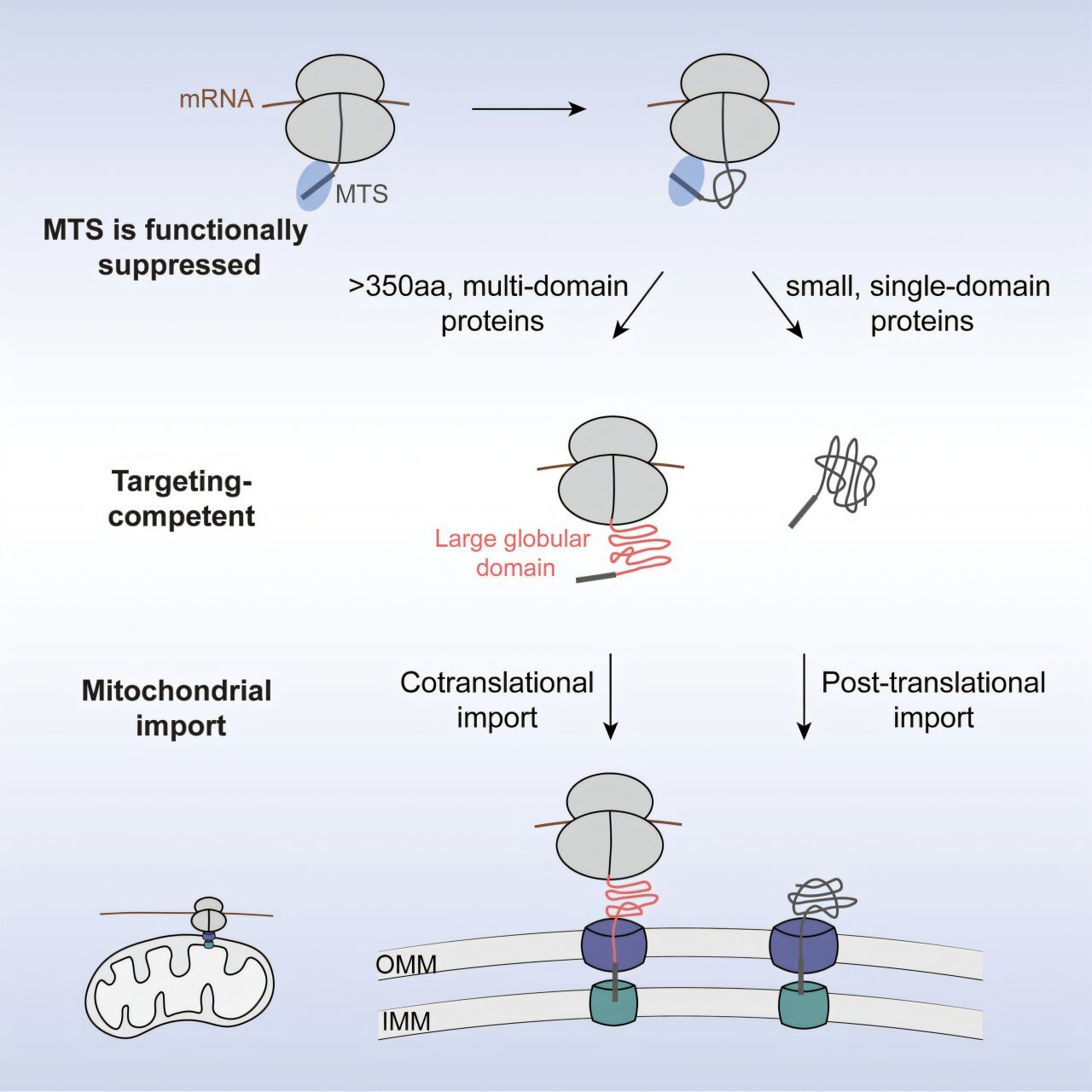
The key lies in protein folding. Proteins aren’t just strings of amino acids; they must fold into precise three-dimensional shapes in order to work. Some proteins fold easily, almost like origami following simple creases. Others, however, are large and complex, requiring distant parts of the chain to come together in just the right way. These complicated proteins face a special problem: if they finish folding outside the mitochondrion, they may become too bulky or tangled to squeeze through the narrow import channels of the mitochondrial membrane.
This is where Caltech chemist Shu-ou Shan and her team made a remarkable discovery. They found that up to 20% of mitochondrial proteins are not imported after translation, as the old model suggested, but during translation itself. In other words, the ribosome begins making the protein, and before the chain is even finished, the partly formed structure is already being delivered into the mitochondrion.
This process—called cotranslational import—acts like an early boarding pass for the most difficult-to-fold proteins, ensuring they don’t get stuck outside in the cytosol where they would clog the system.
The Boarding Pass and the Lock
But how does the cell know which proteins need to be imported early?
Most mitochondrial proteins contain a targeting sequence—a kind of molecular address label that directs them to the mitochondria. But Shan’s group discovered that this label isn’t enough to guarantee early delivery. Instead, the system waits for a second signal.
That second signal is the appearance of the first large protein domain—a foldable structural unit that emerges from the ribosome like the first recognizable shape in a puzzle. Once this domain shows up, it acts like the unlock code to a suitcase containing the boarding pass. Only then is the protein cleared to enter mitochondria during translation.
Zikun Zhu, Shan’s former graduate student and lead author of the study, put it memorably: “The targeting sequence is the boarding pass, but the large domain is the code to open the suitcase.”
In elegant experiments, the researchers even transplanted these large domains onto other proteins. Suddenly, those proteins—which normally waited until after translation to enter mitochondria—were rerouted for early, cotranslational import. It was proof that the domain itself serves as a transferable signal.
A Different Pathway, A Different Future
This discovery is more than just a technical detail of cell biology. It reveals that cotranslational targeting to mitochondria is completely different from how proteins are delivered to other organelles, like the endoplasmic reticulum. It suggests that cells evolved a uniquely sophisticated system to safeguard their most delicate, complex proteins.
And the implications reach beyond curiosity. By learning how to manipulate this process, scientists may one day be able to correct mitochondrial disorders, which are often caused by faulty protein import. These conditions, though rare, can lead to devastating problems in energy-hungry tissues like the brain, muscles, and heart. Understanding the rules of protein targeting could also inspire new therapies or even synthetic biological systems where proteins are engineered with custom delivery signals.
The Poetry of Cellular Precision
There is something poetic in this discovery. A billion years ago, two very different organisms entered into a partnership. That partnership became the mitochondrion, an organelle so essential that life as we know it could not exist without it. Today, the descendants of that ancient bacterium still rely on their host for help, receiving a steady supply of proteins carefully folded and delivered with exquisite precision.
The fact that our cells evolved such a multilayered, almost artistic system to import proteins speaks to the elegance of biology. It shows us that life doesn’t just solve problems—it solves them in ways that balance physics, chemistry, and history.
When we look closely, even the smallest details of the cell tell a story of survival, cooperation, and creativity. Mitochondria are not just powerhouses; they are living reminders that life’s greatest strength lies in connection, adaptation, and the ability to fold complexity into harmony.
More information: Zikun Zhu et al, Principles of cotranslational mitochondrial protein import, Cell (2025). DOI: 10.1016/j.cell.2025.07.021