For over three decades, the world of chemistry has waited for a moment like this. Since the discovery of fullerenes in 1990 — those elegant, soccer-ball-shaped molecules of carbon — scientists have dreamed of coaxing another exotic form of pure carbon into stable existence. Now, researchers at the University of Oxford’s Department of Chemistry have done just that, unveiling a new kind of carbon allotrope, one stable enough to study under ordinary laboratory conditions.
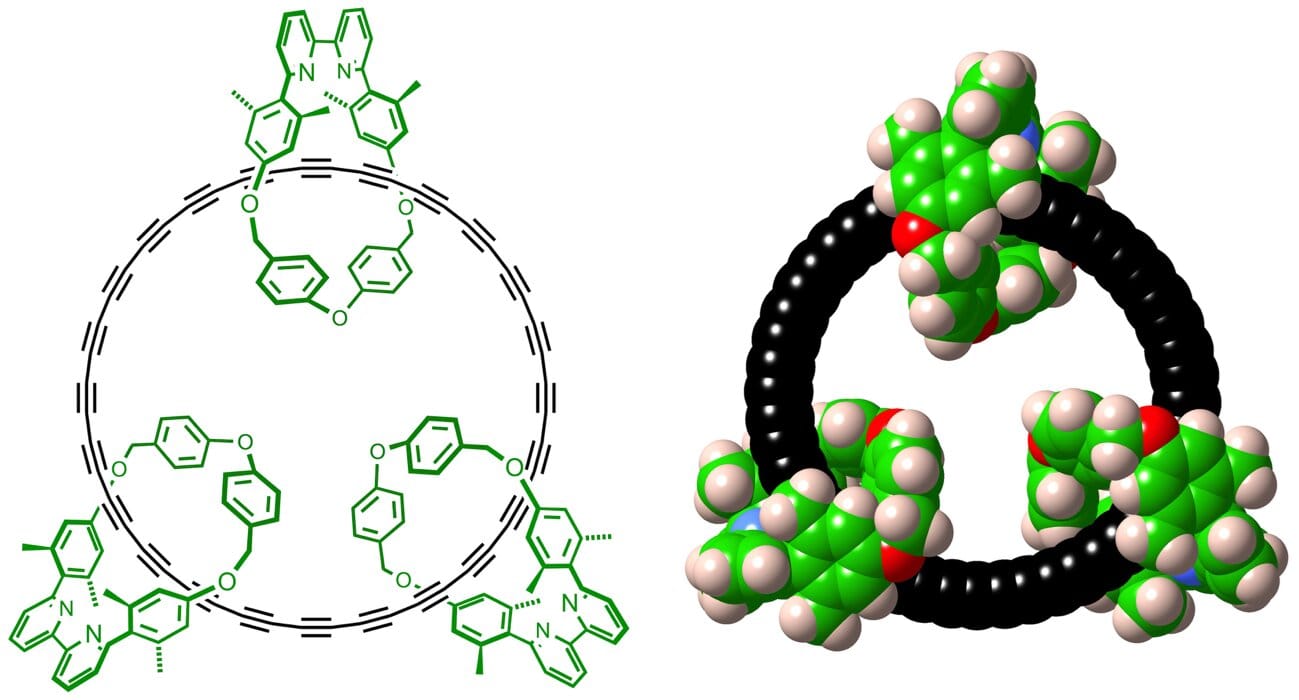
The achievement, published in Science, is being hailed as a milestone in molecular design. At its heart lies cyclo[48]carbon, a ring of 48 carbon atoms arranged in perfect symmetry, protected by three larger molecular loops known as macrocycles. This unusual threading, which chemists call a catenane, gives the fragile carbon ring the shielding it needs to survive in solution at room temperature — a feat once thought almost impossible.
Building Carbon’s New Identity
Carbon is a shape-shifter of the periodic table. It is the element of diamonds and graphite, of nanotubes and fullerenes, of life itself. Each form, or allotrope, reveals a different side of carbon’s versatility. But cyclocarbons — molecular rings made entirely of carbon atoms linked in sp¹ bonds — have long been elusive.
Until now, they could only be glimpsed under extreme conditions: isolated in the gas phase or chilled to just a few degrees above absolute zero. At room temperature, they quickly fell apart. That fragility made them more a theoretical curiosity than a usable material.
What makes the Oxford team’s work extraordinary is that they have stabilized cyclo[48]carbon in solution at 20°C, with a half-life of 92 hours. For chemists, this is like coaxing a soap bubble to hold its shape in the open air — delicate, fleeting, and yet breathtakingly real.
The Art of Molecular Threading
So how did they do it? The secret lies in molecular threading. By passing the C48 carbon ring through three protective macrocycles, the researchers created a catenane — a mechanically interlocked molecule. These “molecular bodyguards” prevent unwanted reactions by shielding the delicate ring from chemical attacks.
The team also made deliberate design choices: selecting a larger cyclocarbon to reduce strain, and carefully tuning the chemical “unmasking” step that transforms a protected precursor into the final cyclocarbon structure. It was a balance of precision, patience, and intuition.
The result: a molecular species that can finally be probed with the full toolkit of chemistry.
Seeing the Invisible
Characterization was as important as synthesis. Using mass spectrometry, NMR, UV-visible, and Raman spectroscopy, the researchers confirmed the structure of the cyclocarbon catenane.
One signal stood out: in 13C NMR spectroscopy, the molecule produced a single, sharp resonance for all 48 carbon atoms. This was the fingerprint the team had hoped for — strong evidence that every carbon atom sits in an identical environment, just as theory predicted.
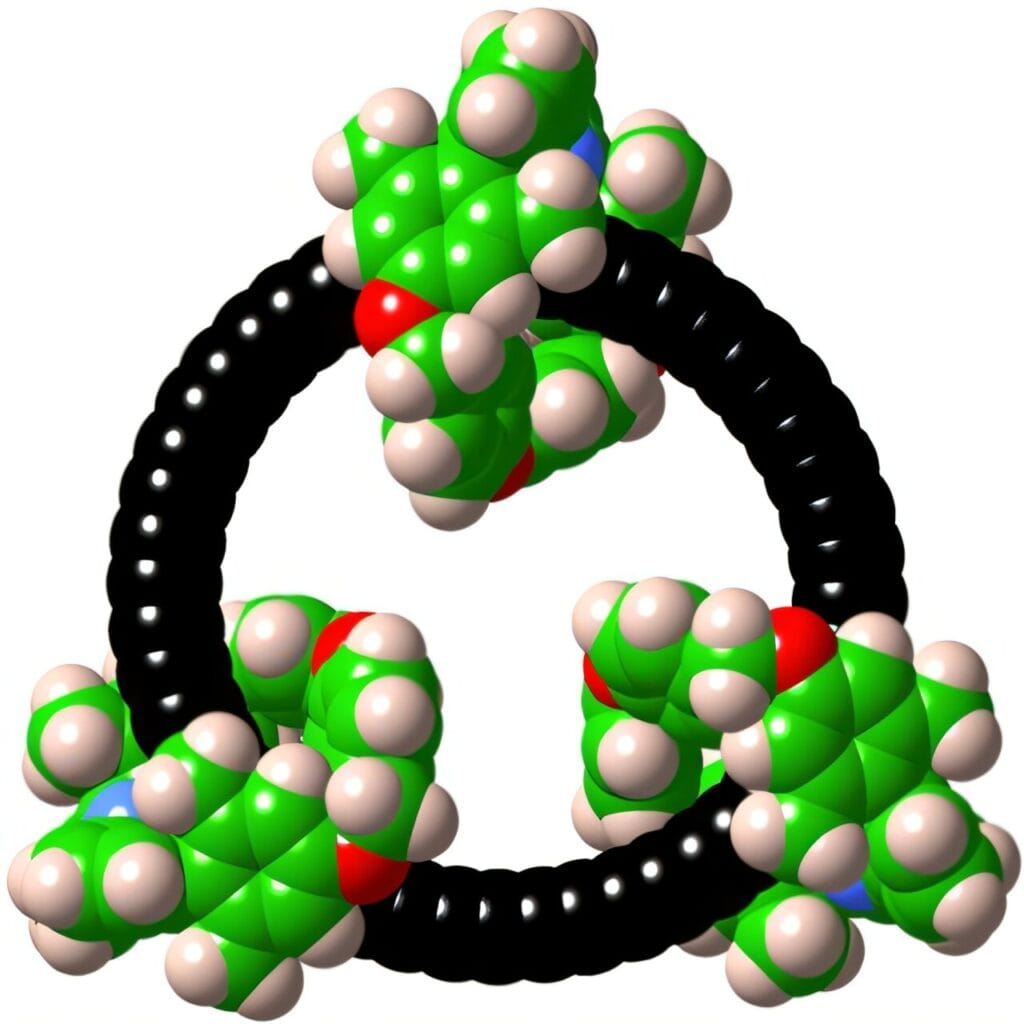
For the first time, a pure cyclocarbon had revealed its secrets not in fleeting traces, but in the full clarity of spectroscopy at room temperature.
A Human Journey of Persistence
Behind the breakthrough lies a story of determination. Lead author Dr. Yueze Gao described the achievement as a fundamental step:
“Achieving stable cyclocarbons in a vial at ambient conditions is a fundamental step. This will make it easier to study their reactivity and properties under normal laboratory conditions.”
For senior author Professor Harry Andersen, the discovery was the culmination of a decade-long effort that often seemed destined for failure.
“The original grant proposal was written in 2016, based on preliminary results from 2012–2015. It is satisfying to have reached this point, because there were many times when the goal seemed unrealistic and unachievable. This work would not have been possible without the outstanding facilities for NMR spectroscopy in the Department of Chemistry at Oxford.”
Their words echo a truth familiar to many scientists: discovery is not a straight line but a path marked by setbacks, revisions, and occasional flashes of triumph.
Why This Matters
Beyond the immediate thrill of synthesis, stable cyclocarbons open doors to new questions. How do these exotic molecules react with other species? Can their unique electronic properties be harnessed in nanotechnology, electronics, or materials science?
The history of carbon allotropes suggests that today’s curiosities can become tomorrow’s revolutions. Fullerenes, once laboratory novelties, are now used in everything from solar cells to drug delivery. Could cyclocarbons follow the same trajectory?
For now, what matters most is possibility. Chemists around the world now have a new tool, a new puzzle piece in the grand mosaic of molecular science.
A Future Written in Carbon Rings
Carbon, more than any other element, defines life and technology. With every new allotrope, we gain not just another structure, but another way of imagining the universe’s possibilities.
The Oxford team’s cyclo[48]carbon catenane is not merely a molecule — it is a promise. A promise that the boundaries of chemistry are not fixed, that even the most fragile forms of matter can be coaxed into existence with enough ingenuity.
And in that fragile ring of carbon atoms, spinning quietly in solution, lies a glimpse of the future of chemistry — bold, intricate, and full of wonder.
More information: Yueze Gao et al, Solution-phase stabilization of a cyclocarbon by catenane formation, Science (2025). DOI: 10.1126/science.ady6054. www.science.org/doi/10.1126/science.ady6054