Imagine a winter morning by a frozen lake. The surface glitters with a sheet of ice, hard enough to walk on, while fish swim calmly beneath in liquid water. It seems ordinary. Yet what you’re witnessing is one of the strangest phenomena in nature: ice floats.
Most substances get denser as they freeze—solids sink in their own liquids. But not water. Ice is lighter than water. This anomaly isn’t just a quirk; it’s a miracle that helps life on Earth survive. The chemistry behind this strange behavior reveals the profound complexity of one of the most essential substances in the universe.
Water seems simple—two hydrogens and an oxygen—but its properties are anything but. From the unique shape of its molecules to the bonds that hold them together, water challenges our expectations at every turn.
So why does ice float? To answer that, we need to dive deep into the molecular realm, where geometry, polarity, and hydrogen bonding reign. Let’s embark on a journey through the invisible world of atoms and forces to uncover the secrets behind floating ice—and why life itself may depend on this icy exception.
Molecules: The Lego Blocks of Matter
At its core, chemistry is the science of how atoms come together to form molecules. Every substance you’ve ever seen or touched—wood, air, stone, metal, blood—is made up of these tiny units.
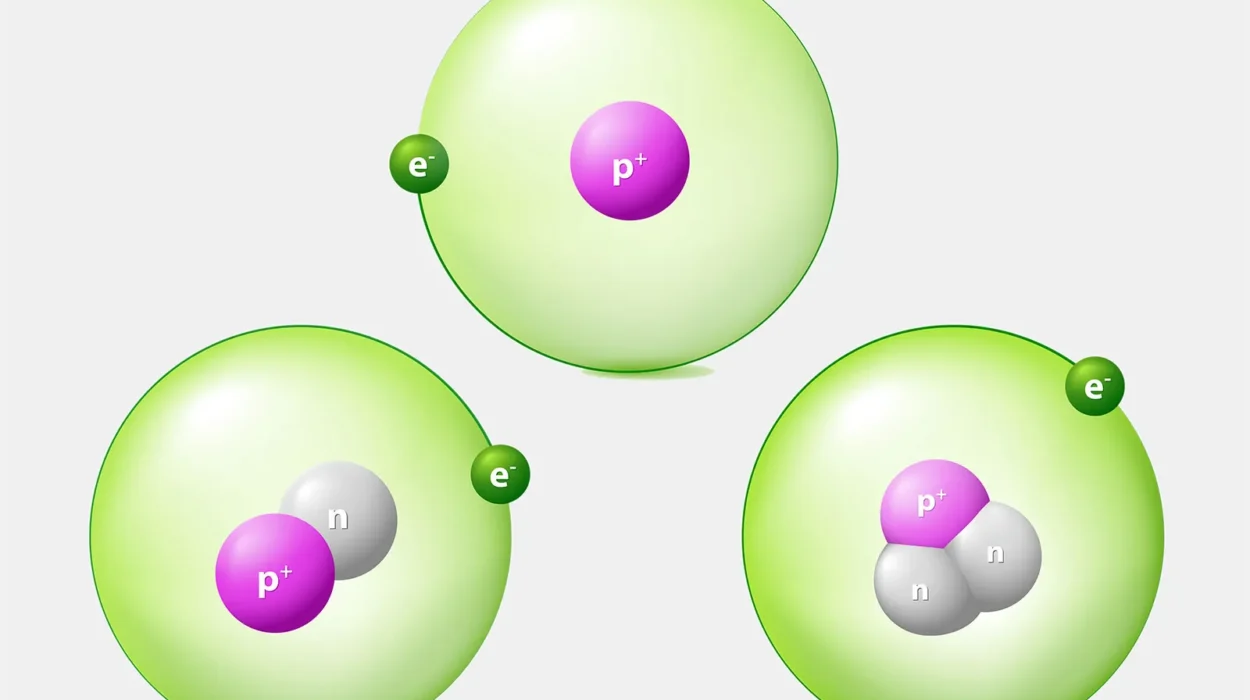
Water’s chemical formula, H₂O, means each molecule contains two hydrogen atoms and one oxygen atom. That sounds simple, but the way these atoms connect makes all the difference.
Oxygen is a greedy atom. It’s highly electronegative, meaning it pulls electrons toward itself. Hydrogen, by contrast, is easygoing. When oxygen and hydrogen form a bond, the electrons spend more time near the oxygen. This gives oxygen a slight negative charge, while each hydrogen becomes slightly positive.
This uneven distribution of charge makes water a polar molecule—like a tiny magnet, with a positive and a negative end. This polarity is key to nearly every unusual property water has, including why ice floats.
But there’s more to the story. The shape of the molecule also matters.
The Bent Shape That Changed Everything
Picture a water molecule. You might think it looks like a straight line: H–O–H. But it’s not.
Water molecules have a bent or V-shaped structure, with the hydrogen atoms positioned at an angle of about 104.5° relative to each other. This angle arises from the electron cloud around oxygen. Oxygen has two lone pairs of electrons not involved in bonding, and these push down on the hydrogen atoms, bending the molecule.
This bent shape ensures that one side of the molecule (where the hydrogens sit) is more positive, while the opposite side (where the lone electron pairs are) is more negative. The molecule now has two poles—a dipole—and that creates opportunities for a special kind of connection called a hydrogen bond.
Hydrogen Bonds: Tiny But Mighty
Hydrogen bonds are weak attractions that occur when a hydrogen atom in one molecule is attracted to an electronegative atom (like oxygen) in another molecule. These are not true chemical bonds—they’re more like magnetic tugs—but they play an enormous role in the behavior of water.
In liquid water, each molecule can form up to four hydrogen bonds with neighboring molecules. These bonds are constantly forming, breaking, and reforming as the molecules move and jiggle around, especially with added heat.
This network of fleeting connections gives water its remarkable properties: high surface tension, high specific heat, and cohesion.
But here’s the twist: when water freezes, the hydrogen bonds lock into place. And the way they lock determines everything.
Freezing: The Birth of Structure
As water cools, the molecules move more slowly. At around 0°C (32°F), they start to form a crystalline lattice—a rigid, ordered structure stabilized by hydrogen bonds. This structure forces the molecules to spread out and arrange themselves at fixed distances.
The result? The molecules are farther apart in ice than in liquid water. And when molecules spread out, the substance becomes less dense.
That’s the answer to why ice floats: ice is less dense than liquid water because of the open hexagonal lattice formed by hydrogen bonds in its solid state.
It’s a counterintuitive twist: freezing a liquid makes it expand. Only a few other substances behave this way—silicon, gallium, and bismuth among them—but water is by far the most important.
Now that we understand the structure, let’s consider the consequences.
The Miracle of Floating Ice
When ice forms on the surface of a pond, lake, or ocean, it floats. If ice sank, cold environments would freeze from the bottom up. Aquatic life would be doomed. But because ice stays on the surface, it creates an insulating barrier that protects the water below.
This insulating layer is why fish can survive under frozen lakes in winter. It’s why entire ecosystems persist even in polar climates. Ice’s buoyancy stabilizes Earth’s climate and keeps oceans habitable. Without it, Earth might have been an entirely frozen planet.
So in many ways, life on Earth owes its existence to this strange property of water. Floating ice may have protected early microbes in Earth’s ancient oceans, giving them the time and shelter needed to evolve into everything we know today.
Water’s Other Oddities: A Liquid Like No Other
While floating ice is water’s most famous anomaly, it’s far from the only one. Water defies expectations in dozens of ways, all due to the interplay of hydrogen bonding, polarity, and molecular structure.
For instance, water has:
- High surface tension, allowing insects to walk on it.
- High specific heat, so it resists temperature change—moderating Earth’s climate.
- High heat of vaporization, meaning it takes a lot of energy to boil, aiding in cooling (like sweating).
- Density maximum at 4°C, so colder water rises above warmer water just before freezing—helping lakes turnover nutrients.
All of these traits come from the same deep source: the molecular geometry of water and the behavior of hydrogen bonds.
What If Water Behaved Normally?
Let’s play a thought experiment: What if water behaved like most other substances—becoming denser as it solidified?
First, lakes and oceans would freeze from the bottom up. Ice would sink, layer upon layer, until entire bodies of water froze solid. Seasonal melting wouldn’t be enough to reverse the process. Eventually, Earth’s water would become locked in ice.
Climate would become far more volatile. Without the high specific heat of liquid water, temperature swings would be more extreme. Storms, droughts, and wild temperature shifts would dominate the weather.
On a larger scale, water’s ability to regulate heat on Earth would vanish. Without oceans acting as thermal buffers, much of the planet would be uninhabitable.
And, of course, life as we know it wouldn’t exist—or if it did, it would look very different.
The Cosmic Perspective: Is Water Unique?
While Earth is rich in water, it’s not alone in the cosmos. Many moons in our solar system—like Europa, Enceladus, and Ganymede—have subsurface oceans. Exoplanets with water vapor in their atmospheres have already been detected. Could they have floating ice too?
That depends. The behavior of water is shaped by universal laws of chemistry and physics, so ice should float elsewhere, too. In fact, scientists often use the presence of water—and especially its phase transitions—as a key indicator when searching for life on other planets.
But water’s quirks are not guaranteed. Slight variations in atmospheric pressure, salinity, or molecular composition could tip the balance. On a high-pressure planet, for example, ice may sink.
So while floating ice may be common, it’s not guaranteed. Our planet might be enjoying a rare sweet spot in cosmic chemistry.
Supercooled Water and Exotic Ices
The story of ice gets even weirder when we consider its many forms. Most people know only one kind of ice—the familiar crystalline type that floats in your drink. But scientists have identified over 20 types of ice, formed under different temperatures and pressures.
There’s:
- Ice I (our common ice),
- Ice II to Ice XV, denser and more complex forms found in labs or deep planets,
- Amorphous ice, found in space, with no regular structure.
Then there’s supercooled water—a state where water stays liquid below its freezing point. In pure conditions, water can remain liquid down to –40°C, forming ice only when disturbed or seeded with particles.
These exotic forms help scientists understand extreme environments, like the interiors of icy moons or comets, and shed light on how water behaves under pressure—literally and figuratively.
Why This Matters Beyond Chemistry
So why should we care about the chemistry of ice floating? Because it links the microscopic to the macroscopic, the simple to the sublime.
A bent molecule, a weak bond, a frozen surface—these small things shape the destiny of worlds.
Understanding why ice floats helps us appreciate how finely tuned our planet is for life. It reminds us that complex outcomes can arise from simple rules. And it shows how the ordinary can be extraordinary when viewed through the lens of science.
This single anomaly—just one property among many—connects quantum mechanics, thermodynamics, environmental science, and biology.
Teaching the Mystery: Ice as a Classroom Portal
Educators love using water to introduce students to chemistry, and floating ice is the perfect hook. It’s familiar, surprising, and leads directly into deeper concepts:
- Molecular structure
- Electronegativity
- Hydrogen bonding
- Phase transitions
- Density and buoyancy
- Thermal properties
Using floating ice as a teaching tool brings chemistry alive. It shows that science isn’t just in textbooks—it’s in our glasses, our lakes, our breath.
Final Reflections: The Elegance of Everyday Wonders
Next time you drop an ice cube into your drink or skate across a frozen lake, pause to consider the hidden architecture beneath your feet. Within each shard of ice is a record of molecular cooperation—of atoms arranging themselves with purpose, of forces aligning just so, to create a structure that defies gravity.
Water, in all its phases, is a marvel. But ice—graceful, buoyant, paradoxical—remains its most poetic form.
It floats not because it wants to, but because the laws of nature demand it. And in doing so, it preserves life, moderates climates, sculpts landscapes, and fuels the great engine of biology.
In the end, the chemistry of floating ice is more than science. It is a quiet symphony of shape, charge, and structure—a story written in atoms, and lived by all of us.