It began as a whisper from the past—a deadly fungus hiding in the shadows of ancient tombs, once feared as a harbinger of the “Pharaoh’s Curse.” Now, nearly a century after mysterious deaths followed the opening of King Tutankhamun’s burial chamber, scientists have reimagined the villainous Aspergillus flavus not as a specter of death, but as a surprising source of healing.
In a breakthrough study led by researchers at the University of Pennsylvania, scientists have turned this notorious fungus into a promising new cancer therapy. Their findings, published in Nature Chemical Biology, reveal a powerful class of molecules—named asperigimycins—that can destroy leukemia cells with the strength of FDA-approved chemotherapy drugs.
“This is what nature does best,” said Sherry Gao, senior author and Presidential Penn Compact Associate Professor of Chemical and Biomolecular Engineering. “Fungi gave us penicillin. This discovery reminds us there are many more medicines hiding in plain sight, waiting to be found.”
A Fungus with a Fatal Reputation
Aspergillus flavus is no stranger to dark headlines. Known for its yellow-green spores and potent toxins, the fungus thrives in soil, decaying crops, and dusty tombs. In the 1920s, after the tomb of Tutankhamun was opened, the deaths of several expedition members sparked worldwide fascination with the idea of a supernatural curse. Later medical analysis suggested that dormant fungal spores, including those of A. flavus, might have played a deadly role.
A similar mystery unfolded in 1970s Poland, when scientists exploring the tomb of King Casimir IV fell ill. Ten of the twelve researchers died within weeks. The culprit? Once again, Aspergillus flavus, whose spores can trigger fatal lung infections in those with weakened immune systems.
But what was once feared as a killer may now become a healer.
A Rare Discovery in the World of Fungi
The Penn-led team wasn’t searching for ancient curses—they were looking for new medicines, particularly a rare and understudied class of natural compounds called RiPPs—ribosomally synthesized and post-translationally modified peptides. These molecules, built by the cell’s protein-making machinery and then chemically modified, have already shown promise as potent therapeutic agents in bacteria. But in fungi, they remain a mystery.
“Purifying fungal RiPPs is difficult,” said Qiuyue Nie, a postdoctoral fellow and first author on the paper. “They’ve been misclassified for years, and we had no clear understanding of how fungi actually make them.”
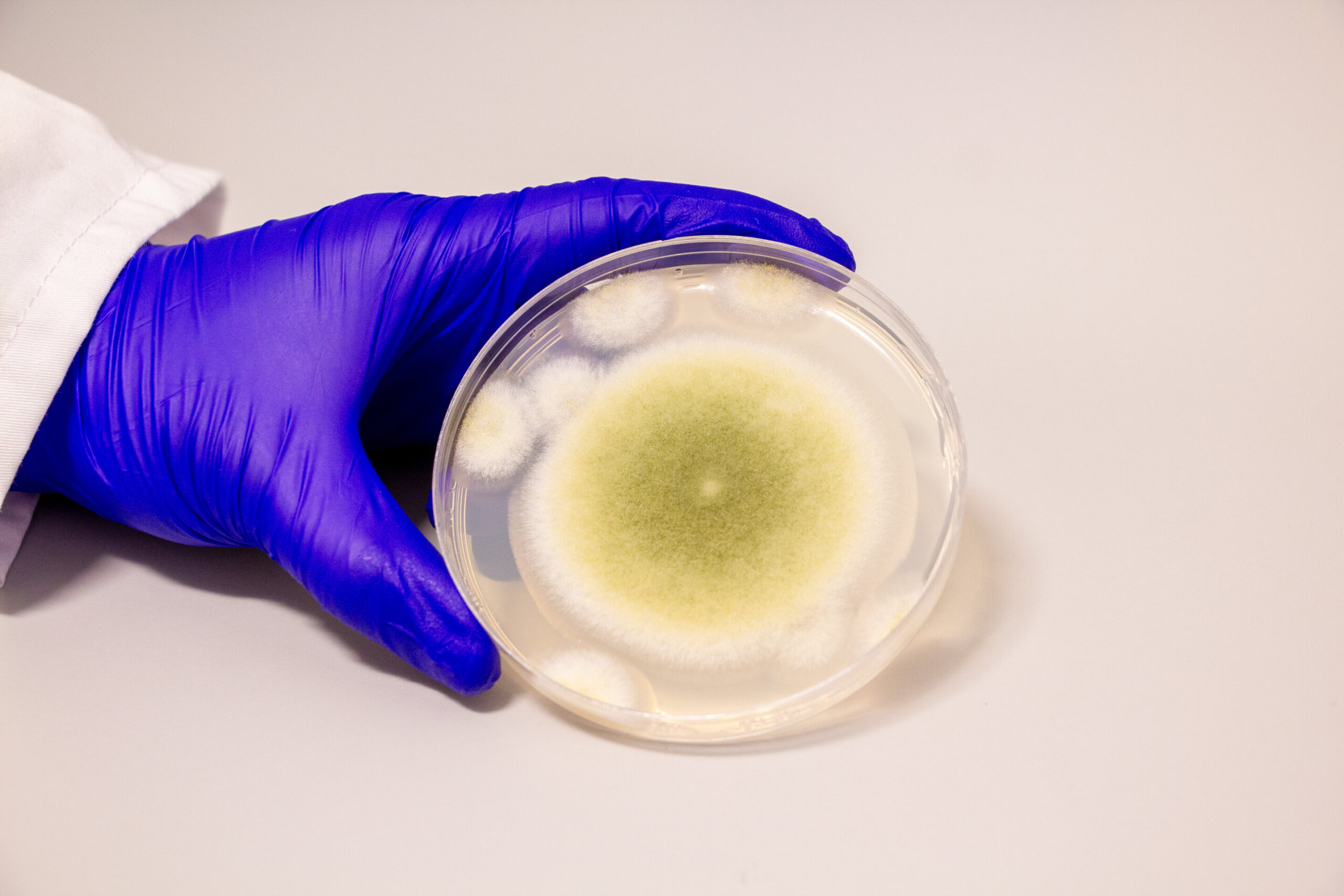
That changed when the team investigated a dozen strains of Aspergillus, hunting for genetic and chemical signatures of these elusive molecules. One species stood out: A. flavus. By silencing a gene believed to be central to RiPP production, they observed a dramatic drop in the fungus’s chemical output—a clear sign they had found their target.
The Birth of Asperigimycins
From the depths of A. flavus, the team extracted four novel RiPP molecules. They had never been described before. Intricately structured with interlocking chemical rings, the compounds were named asperigimycins, a nod to their fungal origin.
When tested against human leukemia cells, asperigimycins showed remarkable potency—especially when one of the variants was modified by adding a fatty molecule found in royal jelly, the nutrient-rich secretion that nourishes queen bees. This lipid-enhanced version performed as well as cytarabine and daunorubicin, two cornerstone drugs used to treat leukemia for decades.
Even more striking? Asperigimycins appeared to target only certain cancer cells, sparing healthy cells from collateral damage. That kind of specificity is a holy grail in oncology, where most treatments are as destructive to the body as they are to cancer.
Unlocking the Gateway to Cells
To understand why some versions of asperigimycins worked better than others, the team began to explore how the molecules entered cancer cells. Their genetic experiments pointed to a single player: a gene called SLC46A3.
“SLC46A3 acts like a gateway,” Nie explained. “It allows asperigimycins to escape the lysosome—basically, a cellular recycling bin—and get into the cell’s main chamber where they can do their job.”
That discovery has implications beyond asperigimycins. Other cancer-fighting peptides—circular compounds similar in shape and structure—often fail because they can’t enter cells efficiently. With the new knowledge that lipid modifications and SLC46A3 help open the gate, scientists now have a powerful tool for designing better, smarter drugs.
Targeting Cell Division Without Collateral Damage
Once inside, asperigimycins disrupt one of cancer’s key strategies: uncontrolled division. The molecules interfere with microtubules, tiny structural rods that help cells divide. Without them, cancer cells can’t reproduce—and they die.
Yet in tests, asperigimycins showed little to no effect on other cancers like breast, liver, or lung, and they didn’t harm bacteria or healthy fungi. This level of cell-type selectivity is rare and invaluable, suggesting that asperigimycins could be developed into targeted treatments with fewer side effects than conventional chemotherapy.
“This is what you want in a drug,” said Gao. “Precision. Power. And safety.”
A Vast Pharmacy Beneath Our Feet
While this discovery is a breakthrough in itself, it may just be the beginning. The researchers identified similar gene clusters in other fungi, hinting at a vast, untapped reservoir of RiPPs that could yield new antibiotics, antivirals, and anticancer drugs.
“Fungi are like a hidden library,” Nie said. “We’ve only just begun to read the first few pages.”
The next step is to test asperigimycins in animal models, and, if the results hold, eventually move toward human clinical trials. It’s a long road, but one that now seems possible—thanks to a killer fungus reborn as a healer.
Nature’s Secrets, Reimagined
In a world increasingly threatened by drug resistance and complex diseases, this study is a vivid reminder of nature’s limitless potential—and humanity’s growing ability to decode it. From the cursed tombs of history to the cancer wards of tomorrow, Aspergillus flavus has traveled a remarkable arc: from feared killer to possible savior.
“Nature has given us this incredible pharmacy,” said Gao. “It’s up to us to unlock it. And with every discovery, we learn not just how to treat disease—but how to imagine entirely new ways of healing.”
Reference: A class of benzofuranoindoline-bearing heptacyclic fungal RiPPs with anticancer activities, Nature Chemical Biology (2025). DOI: 10.1038/s41589-025-01946-9