When a spider spins its web, something extraordinary unfolds. The silk that begins as a liquid quickly transforms into a solid form that, weight for weight, is stronger than steel. This remarkable process occurs at room temperature and involves biodegradable, environmentally friendly polymers. Spiders accomplish this without industrial-scale machinery or harmful chemicals, a feat that has captivated materials scientists for years. Researchers at Carnegie Mellon University are delving deep into the molecular mechanics of spider silk to understand how nature manipulates polymers so efficiently. By mimicking these biological techniques, they hope to revolutionize the industrial processing of plastics.
Polymers, the building blocks of materials like plastics, exhibit unique properties due to their molecular structures. A polymer molecule can take on various forms, such as linear chains resembling strings, interconnected mesh-like networks, or closed-looped rings. These shapes—or “architectures”—play a crucial role in determining a polymer’s properties, including its strength, flexibility, and recyclability. Among these, ring-shaped polymers have emerged as a particularly fascinating subject because they exhibit behavior that could pave the way for creating sustainable and recyclable materials.
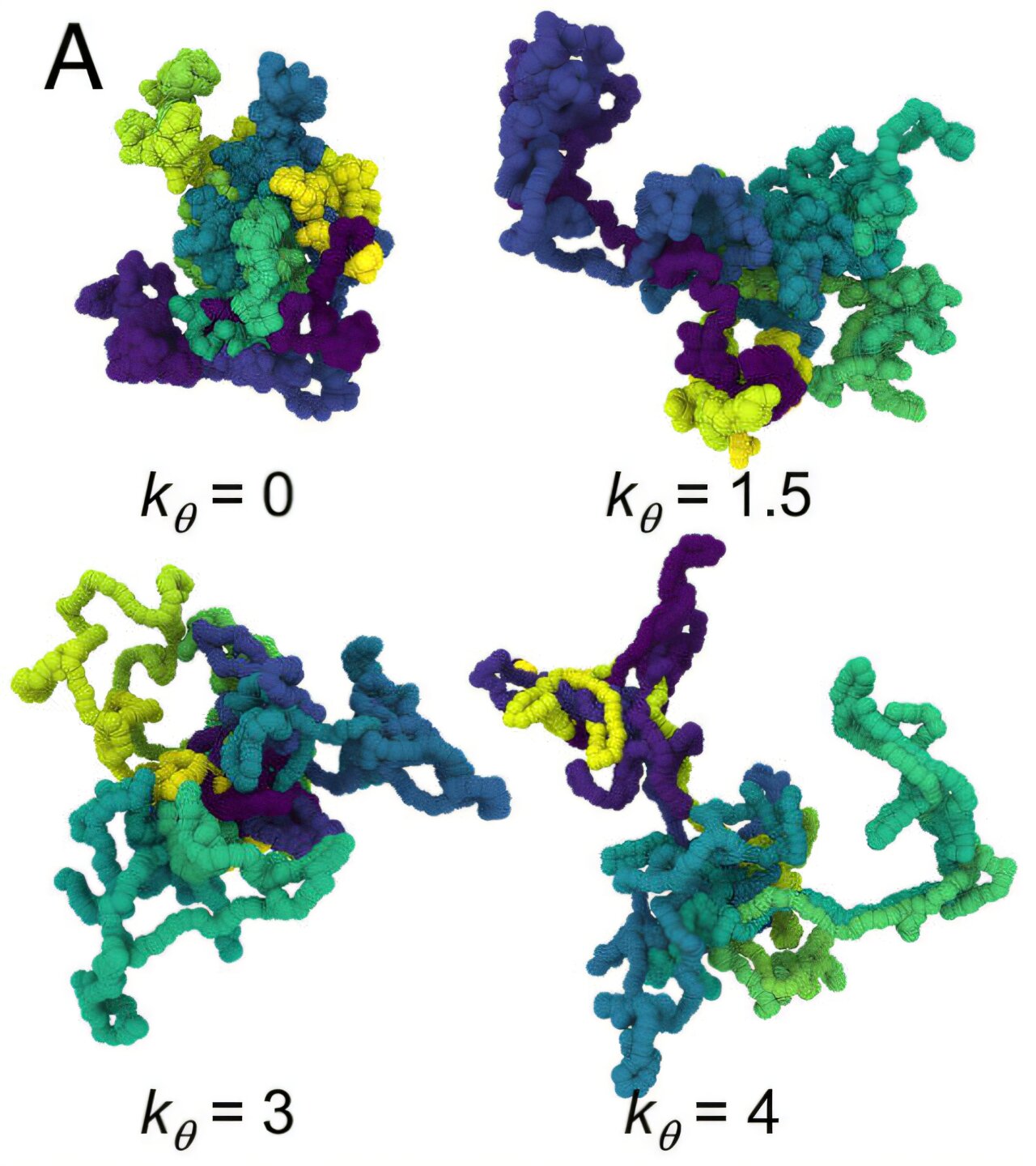
A groundbreaking study conducted by a team of researchers from Carnegie Mellon, Sandia National Laboratories, and the University of Illinois at Urbana-Champaign (UIUC) has provided fresh insights into ring polymers. Their investigation, which involved the most extensive simulation of ring-shaped polymers to date, confirmed long-standing theoretical predictions. The research showed that when ring polymers grow sufficiently long, they spontaneously solidify into a glass-like state. This surprising transition is not a result of cooling, as is typical in polymer science, but rather due to the unique packing dynamics of the closed-ring molecular architecture.
In the study, published in Proceedings of the National Academy of Sciences, the researchers demonstrated that altering a polymer’s shape from open chains to closed rings fundamentally changes how the molecules interact and move within a material. As the rings become longer, the individual chains become increasingly crowded, limiting their ability to move freely. Eventually, the constrained motion causes the material to vitrify—or turn into a glassy solid. This process occurs without altering temperature, highlighting the profound impact that molecular architecture can have on a material’s phase behavior.
“We usually have to cool a sample’s temperature to make molten plastic solidify, but we have found that simply changing the shape of the molecules into rings causes them to slow and vitrify into glass,” explained Thomas O’Connor, an assistant professor of materials science and engineering at Carnegie Mellon.
O’Connor, along with doctoral student Songyue Liu, conducted large-scale molecular dynamics simulations on Department of Energy supercomputers to test these theoretical predictions. This computational effort, which spanned over a year, built upon previous experimental work by the team. In earlier studies, they had synthesized a recyclable polymer material made entirely of ring polymers that unexpectedly vitrified under laboratory conditions. The new simulations not only confirm the experimental findings but also provide a theoretical framework for designing recyclable cyclic polymers with tailored properties.
This research goes beyond its implications for polymer science and has potential applications in the field of biopolymers, particularly those found in living organisms. Biological molecules like proteins and DNA often adopt looped or folded structures that resemble the ring polymers studied in this research. Understanding how these loopy polymer structures crowd together, fold, and interact can provide insights into both materials science and biological functions.
For example, chromosomes within the nucleus of a cell organize into intricate looped structures to efficiently store and manage genetic information. Similarly, folded proteins achieve their functionality by adopting specific three-dimensional shapes. By drawing parallels between synthetic ring polymers and these natural systems, researchers can uncover new principles for designing advanced materials and decoding the mysteries of biological processes.
“Understanding how loopy polymer structures crowd around each other and fold up is insightful for materials science, but it is also important for understanding why living systems use these structures to enable biological functions,” said O’Connor. “There is potential for us to draw parallels between these disciplines to make new discoveries.”
The practical applications of this discovery are manifold. For one, this research could lead to the development of sustainable, recyclable plastics that rely on the unique properties of ring polymers. Unlike traditional plastics, which often degrade into microplastics and pose environmental hazards, these cyclic polymers could offer a cleaner and more efficient alternative. Additionally, by controlling how polymers vitrify and pack, industries could design materials with specific properties tailored to applications ranging from packaging to biomedical devices.
The work also sheds light on fundamental questions in polymer science. For decades, researchers have hypothesized that polymer architecture plays a decisive role in determining material properties, but experimental and computational limitations often made it challenging to test these ideas comprehensively. The Carnegie Mellon team’s large-scale simulations and theoretical advancements bridge this gap, providing a more detailed understanding of how molecular shapes influence phase transitions.
Furthermore, the collaboration between experimentalists and computational scientists underscores the interdisciplinary nature of modern materials research. By combining experimental synthesis with theoretical modeling and computational simulations, the team has created a robust framework for exploring uncharted territories in polymer science. This approach not only enhances our understanding of existing materials but also opens up new avenues for creating innovative ones.
The implications extend beyond the laboratory. As the global demand for plastics continues to rise, finding ways to make these materials more sustainable and environmentally friendly has become an urgent priority. The lessons learned from spider silk, ring polymers, and biological systems offer a roadmap for addressing this challenge. By designing polymers that are not only strong and versatile but also recyclable and biodegradable, scientists can contribute to a more sustainable future.
Reference: Baicheng Mei et al, Unified understanding of the impact of semiflexibility, concentration, and molecular weight on macromolecular-scale ring diffusion, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2403964121