In the quiet hours of the night, while our conscious minds are fast asleep, a different kind of clock continues to tick inside our bodies—one we rarely think about but that might be central to how we age. New research has now revealed that deep within our muscles lies a built-in circadian clock, one that doesn’t just keep time but may hold the key to preventing premature aging. And when that internal timekeeper is thrown off by something as seemingly routine as working the night shift, the consequences could be far more profound than previously imagined.
A groundbreaking study by researchers at King’s College London, recently published in the Proceedings of the National Academy of Sciences, lifts the curtain on a hidden biological drama—where muscle cells follow their own daily rhythms, breaking down damaged proteins while we sleep and keeping our tissues young and strong. But when these rhythms are disturbed, the repercussions ripple through the entire muscular system, accelerating age-related decline in a condition known as sarcopenia.
Muscle Clocks: The Secret Timekeepers of the Body
It has long been known that the body is governed by circadian rhythms—roughly 24-hour cycles driven by the brain’s suprachiasmatic nucleus, which syncs with environmental cues like light and darkness. These rhythms dictate sleep, hunger, body temperature, and hormonal balance. But now, scientists are uncovering a deeper layer of complexity: each organ, and even each type of cell, appears to possess its own autonomous circadian clock.
Muscle cells are no exception. Within their microscopic structures, molecular gears spin in perfect time, orchestrating cycles of protein creation and destruction. The new study finds that this muscle clock plays a vital role in the nightly housekeeping that muscles perform while the rest of the body rests. As we go about our day, muscle fibers accumulate wear and tear—damaged proteins pile up from regular use. At night, the muscle clock kicks into action, activating pathways to degrade and recycle these faulty proteins, effectively resetting the muscle for a new day of function.
This elegant mechanism helps explain why sleep is so restorative. It’s not just about recharging the brain—it’s also when your muscles quietly repair themselves, guided by their internal clocks. But if the rhythm of that clock is thrown into disarray, the consequences can be severe.
Shift Work and Muscle Aging: A Dangerous Connection
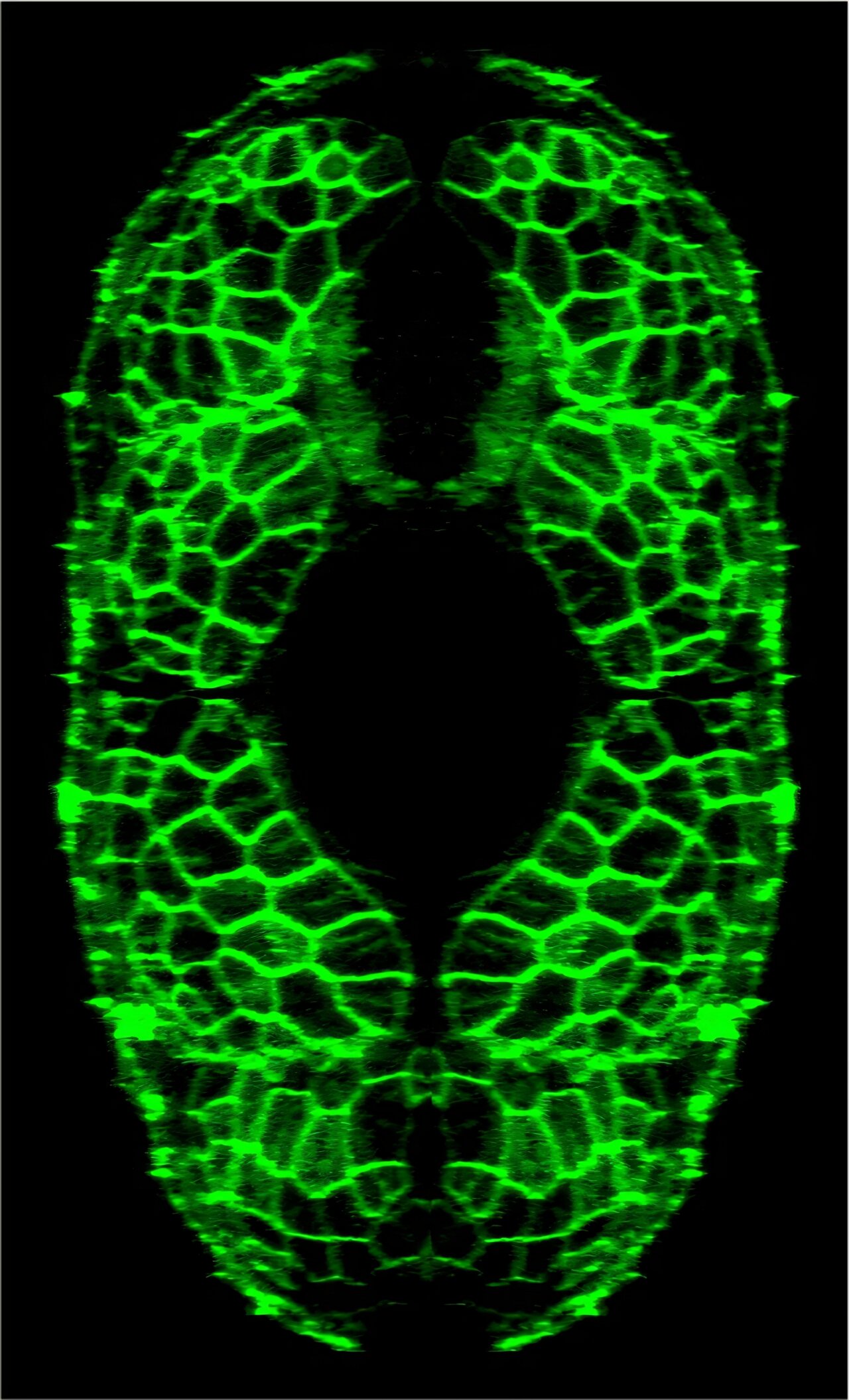
The King’s College team set out to understand exactly what happens when this circadian machinery is disrupted. To do so, they turned to an unlikely hero of biomedical research: the zebrafish.
Zebrafish might seem like an odd stand-in for humans, but biologically, they punch well above their weight. About 70% of their genes have human counterparts, and their transparent bodies make them ideal for observing internal organs and cellular processes in real time. Their muscle structures and responses to circadian regulation closely mimic those found in people, making them a powerful model for aging research.
Lead author Dr. Jeffrey Kelu and his team genetically modified zebrafish to express a malfunctioning version of a key clock protein within their muscle cells. In essence, they “broke” the muscle clock, preventing it from running properly. Then they watched.
For two years—an entire lifetime in the zebrafish world—the team observed how these disrupted fish compared to their normal counterparts. Initially, there was little visible difference. At six months and even one year of age, the fish looked normal, swam just fine, and had average muscle mass. But by two years, a dramatic shift had occurred.
The fish with broken muscle clocks began to exhibit clear signs of premature aging. They were smaller, weighed less, and moved more slowly and less frequently. Their muscles deteriorated, showing hallmarks of sarcopenia, the age-related loss of muscle mass and function that plagues millions of older adults around the world. Essentially, their bodies were aging faster—accelerated not by genetics or disease, but by a disruption in the fundamental rhythm of muscle maintenance.
The Role of Protein Turnover in Aging
At the heart of this aging process lies protein turnover—the continuous process by which cells break down old or damaged proteins and replace them with new, functional ones. This cycle is essential for all tissues but is especially crucial in muscle, which endures constant mechanical stress.
In healthy muscles, the circadian clock ensures that this turnover occurs at the right time—primarily at night when the body is at rest and energy can be devoted to repair rather than action. The new study demonstrated that this rhythmic breakdown and clearance of defective proteins is not just beneficial; it’s essential. When the clock was disrupted, this nocturnal cleansing was impaired. Damaged proteins built up in the muscle fibers, interfering with function and hastening degeneration.
This isn’t just a theoretical concern. Millions of people worldwide work in roles that disrupt their natural circadian rhythms. In the UK alone, approximately four million individuals work night shifts or rotating shifts. They keep hospitals running, ensure public safety, drive delivery trucks, and operate power plants. But the toll of shift work on their bodies is becoming increasingly clear—and now includes the possibility of accelerated muscle aging.
From Fish to Future Therapies
Dr. Kelu emphasizes the broader implications of these findings. “Understanding how circadian disruption contributes to sarcopenia is essential for developing strategies to improve the health and well-being of shift workers,” he said. The hope is that by identifying the molecular gears that keep the muscle clock running smoothly, researchers can find ways to protect it—or even reset it—when it’s thrown off course.
Intriguingly, preclinical studies are already underway to explore drugs that target specific clock proteins. These potential therapies could help restore normal circadian function in muscle tissue, slowing or even reversing the decline associated with disrupted rhythms. This approach represents a promising frontier in preventive medicine, especially for those whose work demands that they operate outside the natural day-night cycle.
The Simplicity and Power of Zebrafish
Professor Simon Hughes, a co-author of the study and a renowned expert in developmental biology, highlighted the significance of using zebrafish for this kind of research. “This work shows how studying something as complicated as muscle growth in a simple system, like a little fish larva, can really teach us something,” he said. “Of course, someone then has to check if it’s also true in people—but at least the fish show us where to look.”
And indeed, the fish are telling us something profound. They are showing us that aging isn’t just about the passage of time—it’s about how our bodies keep time. Or fail to.
A New Perspective on Circadian Health
This study adds to a growing body of research revealing just how critical our internal clocks are to overall health. Disruptions to circadian rhythms have already been linked to a wide range of conditions—from sleep disorders and obesity to cardiovascular disease and certain cancers. Now, we can add accelerated muscle aging to the list.
The implications are vast, not only for shift workers but for anyone whose lifestyle puts them out of sync with natural light cycles. Late-night screen time, irregular sleep patterns, and jet lag may all have cumulative effects on the health of our muscles—and, by extension, our entire bodies.
For employers, healthcare providers, and policymakers, this research underscores the need to take circadian health seriously. Providing strategies for shift workers to better align their sleep and meal times with circadian biology, designing lighting systems that mimic natural cycles, and potentially offering pharmacological support in the future could all become part of a comprehensive approach to mitigating the health risks of modern work schedules.
Clockwork Muscles and the Future of Aging
The idea that each of our muscle cells contains its own clock—a miniature conductor in a symphony of cellular timekeeping—is as beautiful as it is revolutionary. It challenges the traditional view of aging as an inevitable downhill slide and instead opens the door to new ways of preserving strength and vitality well into old age.
By understanding the molecular choreography of the muscle clock, scientists are moving closer to therapies that don’t just treat the symptoms of aging, but its root causes. And perhaps most importantly, they are shining a light on the hidden costs of modern life, where convenience and productivity often come at the expense of biological harmony.
In the end, this research delivers a powerful message: aging is not just about time—it’s about timing. And keeping your body’s clocks ticking in tune may be one of the most effective ways to stay young at heart, and in muscle.
Reference: Kelu, Jeffrey J. et al, Muscle peripheral circadian clock drives nocturnal protein degradation via raised Ror/Rev-erb balance and prevents premature sarcopenia, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2422446122