For millions of people around the world, rheumatoid arthritis (RA) is more than a medical diagnosis — it’s a daily battle. The disease, an autoimmune condition, turns the body’s defenses against itself, leading to painful joint swelling, stiffness, and long-term damage. Over the past few decades, modern therapies have transformed how RA is managed. Yet, about one in three patients still find little relief. Their immune systems remain stubbornly active, as though the disease refuses to let go.
Why does RA persist in some patients despite the best available treatments? This mystery has frustrated scientists and left patients with lingering pain. But new research from Kyoto University may be closing in on an answer — and with it, a new strategy to bring relief to those most in need.
The Double Life of Helper T Cells
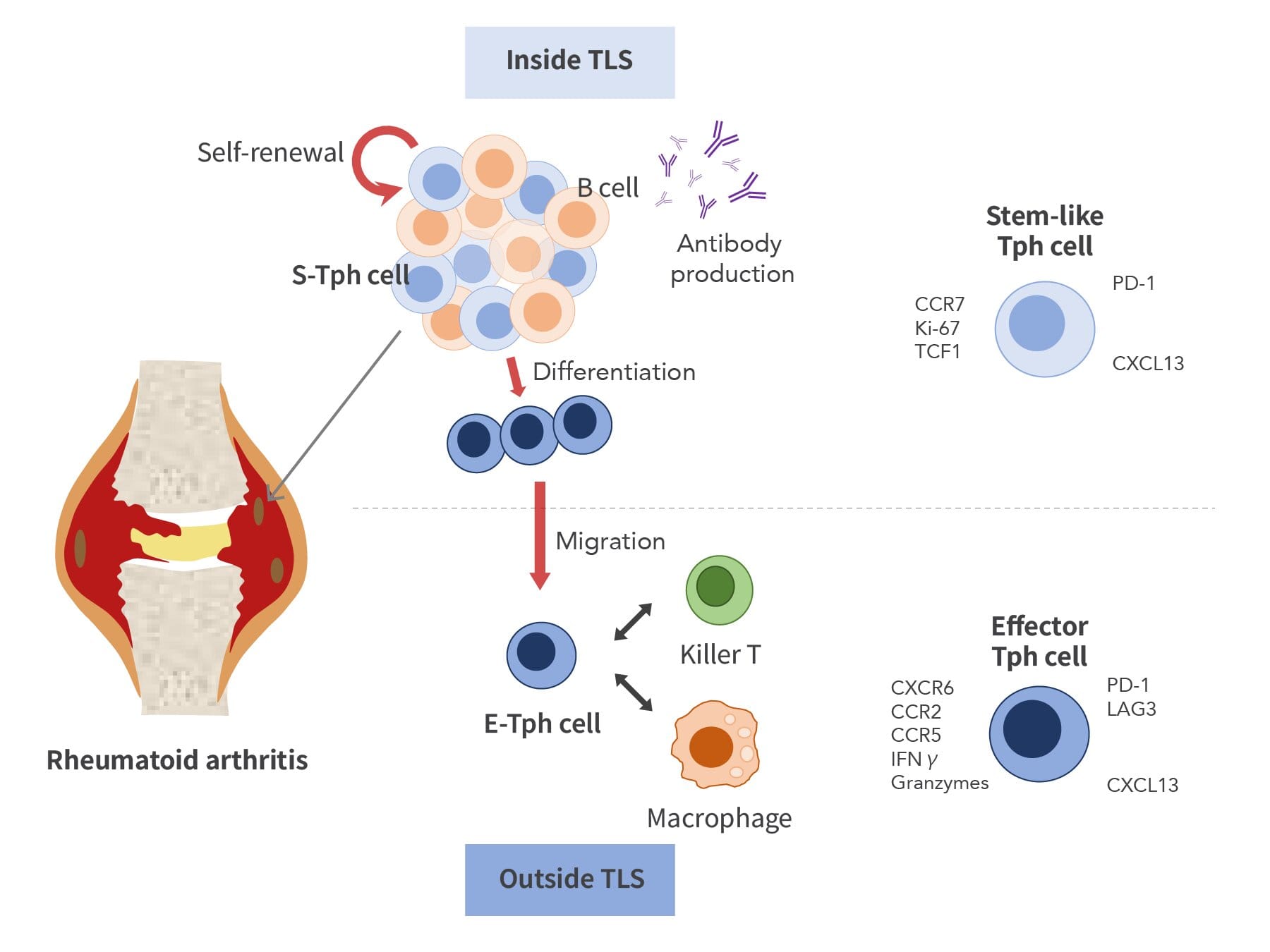
At the center of this breakthrough lies a subset of immune cells known as peripheral helper T cells (Tph cells). These cells act like coordinators in the immune system, guiding other cells — especially B cells, which produce antibodies — into action. Previous studies had shown that Tph cells accumulate in the joints of RA patients, fueling inflammation. But how exactly they persist and maintain the disease was still poorly understood.
The Kyoto team, led by doctoral student Yuki Masuo and immunologists Hiroyuki Yoshitomi and Hideki Ueno, used powerful tools to peer deeper into inflamed joint tissue than ever before. By applying single-cell RNA sequencing and spatial transcriptomics — techniques that can both decode the genetic activity of individual cells and map them in space — the researchers uncovered a hidden dynamic within these destructive immune cells.
Their surprising discovery? Tph cells aren’t all the same. Instead, they exist in two distinct forms: stem-like Tph cells and effector Tph cells.
Inside the Immune “Hubs”
Stem-like Tph cells are the quiet architects of disease. They take up residence in tertiary lymphoid structures (TLSs), small “hubs” of immune activity that form within the inflamed joints of RA patients. In these hubs, stem-like Tph cells multiply and engage in close conversation with B cells. Some of them remain in their stem-like state, capable of dividing again and again. Others transform into effector Tph cells, which then leave the hubs.
Once outside, effector Tph cells act like front-line soldiers — rallying macrophages, killer T cells, and other immune players that drive inflammation. Crucially, these effector cells don’t divide much, meaning their numbers depend on the steady supply from stem-like Tph cells hidden inside the hubs.
This continuous production line may explain why RA inflammation persists even when treatments suppress other parts of the immune system. As long as stem-like Tph cells keep feeding the battle, the disease grinds on.
Why This Discovery Matters
For patients who respond poorly to current therapies, this finding is more than just a scientific detail. It offers a new way of thinking about RA — not just as a runaway immune reaction, but as a system sustained by a root source.
“Because stem-like Tph cells can both self-renew and differentiate, they may represent a root cause of the disease,” said Masuo. In other words, targeting these stem-like cells at the source could cut off the disease’s fuel supply, reducing inflammation at its origin.
If future therapies can safely and specifically disrupt stem-like Tph cells, they may succeed where current drugs fail, bringing relief to the many patients who today remain trapped in cycles of pain and fatigue.
A Window Into the Future of RA Treatment
The Kyoto team’s study, published in Science Immunology, showcases how cutting-edge methods can reveal hidden aspects of disease that were invisible just a few years ago. By combining multi-omics analysis — a layered approach that integrates genetic, molecular, and spatial data — researchers are now able to build a more complete picture of RA at the cellular level.
Professor Ueno emphasized that this is just the beginning. Further work is needed to understand how stem-like Tph cells are maintained, what signals trigger their transformation into effector cells, and how they might be safely targeted without disrupting other critical immune functions. But the path forward is clearer now than before.
Hope Beyond the Science
For patients living with RA, the significance of this research goes beyond technical terms like “stem-like” or “spatial transcriptomics.” It speaks to hope — hope that their pain is not an unchangeable fate, but a puzzle scientists are actively piecing together.
Every discovery like this moves medicine a step closer to treatments that not only manage symptoms but strike at the root of disease. For the one in three RA patients who have felt left behind by current therapies, the Kyoto team’s findings shine a light on new possibilities: fewer flare-ups, less joint damage, and the chance to reclaim a life less defined by pain.
As Masuo put it, “We have uncovered a new aspect of the immune response at the sites of joint damage in RA.” That new aspect may one day be the key to unlocking better treatments — and better lives — for millions.
More information: Yuki Mauso et al, Stem-like and effector peripheral helper T cells comprise distinct subsets in rheumatoid arthritis, Science Immunology (2025). DOI: 10.1126/sciimmunol.adt3955. www.science.org/doi/10.1126/sciimmunol.adt3955