Inside every human body lies a vast ecosystem, teeming with trillions of microbes. For decades, scientists have marveled at how bacteria in the gut shape digestion, immunity, and even mood. But bacteria are only part of the story. Fungi, though far less abundant, live alongside them, quietly influencing the balance of health and disease. Among these fungi, Candida albicans stands out—a yeast that more than half of healthy humans carry in their intestines without issue.
Yet, new research from the University of Illinois Chicago reveals a darker side to this common yeast. In a striking example of cross-kingdom teamwork, C. albicans can actually help a dangerous bacterium, Salmonella enterica serovar Typhimurium, gain a stronger foothold in the gut and spread through the body. The discovery, published in Nature under the title “Commensal yeast promotes Salmonella Typhimurium virulence”, shows just how deeply interconnected our microbial companions truly are.
Salmonella: A Persistent Global Threat
Salmonella Typhimurium is one of the most notorious foodborne pathogens, responsible for an estimated 100 million infections worldwide each year. Most healthy people who contract it endure days of diarrhea, abdominal cramps, and fever before recovering. But for individuals with weakened immune systems, the bacterium can break through the intestinal barrier, spreading to the bloodstream, liver, or spleen—a potentially life-threatening scenario.
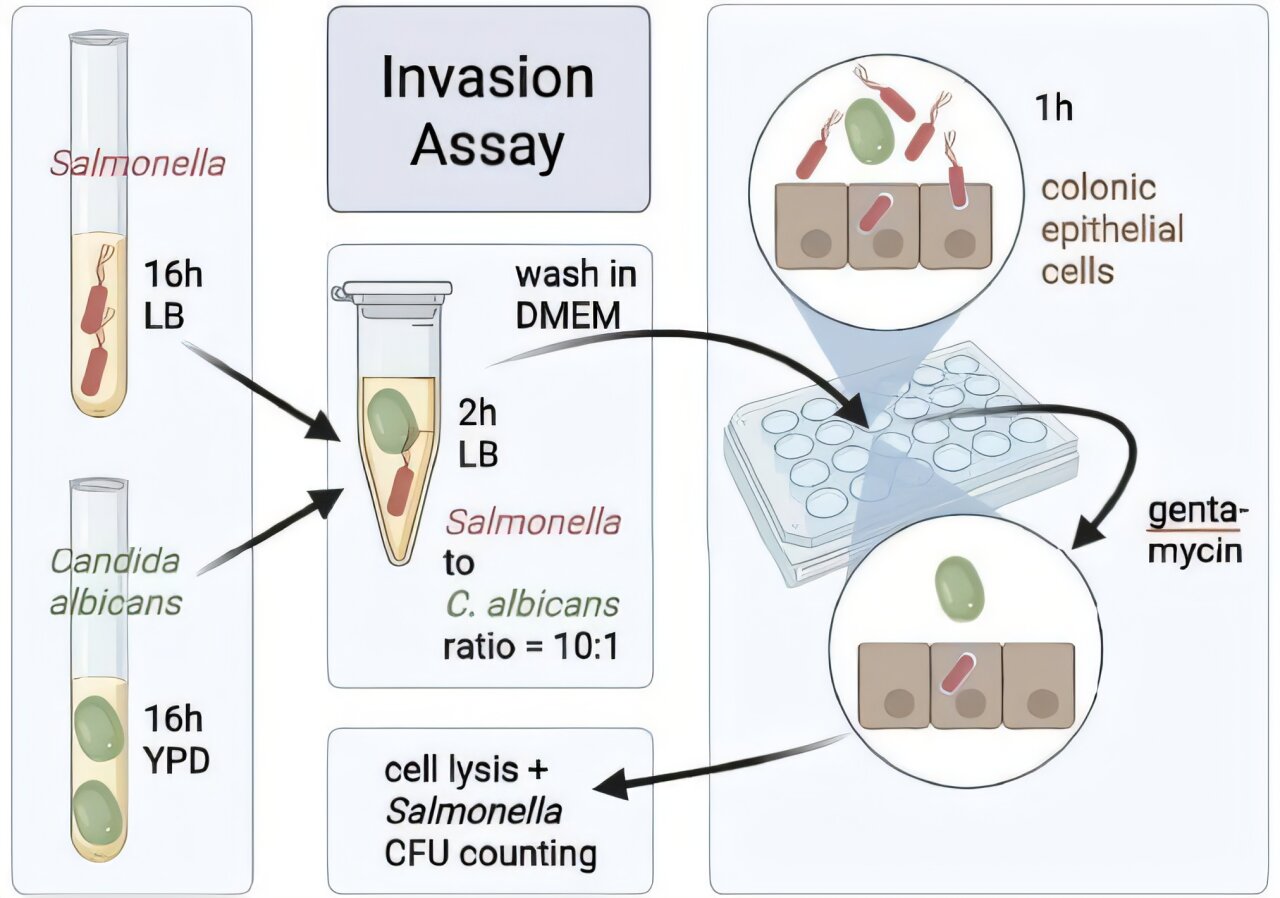
To cause disease, Salmonella must do two things at once: outcompete the crowded community of microbes already living in the gut and activate its specialized invasion machinery, a set of genes known as SPI-1. These genes allow the bacterium to slip into intestinal cells and spread deeper. Normally, the host immune system pushes back fiercely, limiting the damage. But the new study shows that when Candida albicans is present, the balance shifts in Salmonella’s favor.
A Yeast with Two Faces
On its own, C. albicans usually behaves like a harmless passenger. It is found in the mouths, guts, and genital tracts of humans and other mammals, quietly coexisting with its host. But under certain conditions—such as antibiotic use, immune suppression, or chronic inflammation—it can transform. Switching from its round yeast form into invasive hyphae, it is capable of damaging tissues and causing serious infections.
Even when it is not directly causing harm, C. albicans seems to have a knack for exploiting trouble. Previous studies linked it to inflammatory bowel diseases like Crohn’s, where its presence appears to worsen symptoms. Both Candida and Salmonella thrive in inflamed guts, so it was plausible that they might encounter one another inside patients. The UIC-led team asked a deeper question: Could the fungus actively help the bacterium become more dangerous?
The Arginine Connection
The researchers uncovered an elegant molecular dialogue between the two microbes. When Salmonella comes into contact with C. albicans, a bacterial protein called SopB flips a genetic switch inside the fungus. In response, Candida begins producing and releasing large amounts of arginine, an amino acid.
For Salmonella, arginine is more than food—it is fuel for its invasion program. The bacterium senses the arginine and activates SPI-1 genes, priming itself to penetrate intestinal cells. At the same time, arginine seems to calm the host’s immune alarms, lowering signals like IL-17, CXCL1, and interferon gamma. The result is a perfect storm: a bacterium with heightened invasive power and a host with a muted defense.
Mice colonized with both microbes had higher levels of Salmonella in their intestines and more bacterial spread to organs such as the spleen and liver. These animals also lost more weight, a clear marker of illness severity. In lab experiments, Candida boosted Salmonella’s ability to enter cultured human colon cells, and the effect disappeared when fungal arginine production was disabled.
Experiments that Tell a Story
The team tested this interplay across multiple models. In some experiments, mice received antibiotics to disrupt their gut flora, while in others, they carried natural microbiota. Some were germ-free, others colonized with a minimal eight-member microbial community. Across these varied settings, the pattern held: where Candida and Salmonella met, invasion and spread intensified.
To isolate the mechanism, scientists supplemented mice with arginine in their drinking water. Remarkably, this alone mimicked the effect of Candida, boosting Salmonella’s spread and suppressing inflammation. Adding lysine, a competitor amino acid, partly reversed the outcome. This confirmed that arginine was the critical mediator.
Genetic tinkering strengthened the case. Salmonella mutants lacking the arginine transporter failed to benefit from Candida. Meanwhile, fungal strains unable to produce arginine lost their ability to empower Salmonella—until researchers restored the missing genes. The chain of evidence painted a clear picture: SopB-driven arginine production in Candida is the linchpin of this microbial alliance.
A Subtler Kind of Sabotage
What makes this finding so striking is that Candida albicans itself does not trigger gut inflammation in mice. On its own, it sits quietly. But when paired with Salmonella, it acts almost like a backstage accomplice—quieting the immune system, feeding the invader, and making it easier for the bacterium to slip past defenses.
In humans, the clinical relevance is still emerging. A study in Cameroon found that patients colonized with Candida were four times more likely to suffer recurrent typhoid or paratyphoid infections. While not definitive, the link suggests that fungal colonization could be an overlooked factor in bacterial disease risk.
What This Means for Human Health
These findings expand our understanding of the gut microbiome in a powerful way. For years, research has focused mainly on bacteria, given their abundance and influence. But fungi—though fewer in number—can tilt the balance dramatically. They are not mere bystanders; they are active players shaping infection outcomes.
If Candida colonization increases vulnerability to Salmonella, it raises urgent questions. Should antifungal treatments be considered in high-risk patients, such as those who are immunocompromised or living in regions where typhoid is endemic? Could manipulating amino acid availability in the gut offer a novel therapeutic strategy? And more broadly, how many other cross-kingdom partnerships are quietly shaping health and disease in ways we have yet to uncover?
The Bigger Picture: A Living Ecosystem
Our bodies are not just human; they are ecosystems where bacteria, fungi, viruses, and other microbes constantly interact. Sometimes these interactions protect us—commensal bacteria can block pathogens from attaching to the gut lining, or prime our immune systems for defense. But as this study shows, sometimes microbes conspire against us, turning harmless neighbors into unwitting accomplices of disease.
The work from UIC scientists underscores the need to see the microbiome as more than bacteria. The “mycobiome”—the community of fungi within us—may be smaller, but its influence is mighty. Understanding these complex webs of interaction is essential if we are to develop therapies that target infections at their ecological roots.
A New Frontier in Infection Biology
The discovery that Candida albicans fuels Salmonella virulence through arginine release is more than just a fascinating detail. It signals a new way of thinking about infectious disease: one that looks beyond pathogens in isolation and instead considers the entire community in which they live.
It reminds us that illness is rarely a simple battle between a microbe and its host. More often, it is a tangled drama involving multiple species, competing interests, and chemical conversations that span kingdoms of life. By decoding these conversations, we may uncover new strategies to outsmart pathogens—using knowledge, not just drugs, as our defense.
Conclusion: A Subtle but Dangerous Partnership
What began as a routine question about how fungi and bacteria coexist in the gut has revealed a profound truth: even ordinary microbes can tip the scales of health and disease in surprising ways. Candida albicans, long known as a benign passenger, can quietly empower one of the world’s most dangerous bacteria by offering it an amino acid and dampening our defenses.
This partnership highlights both the fragility and resilience of our internal ecosystems. It is a reminder that to truly understand—and protect—human health, we must listen not just to the loudest voices in the microbiome but also to the whispers of overlooked companions like fungi.
The story of Candida and Salmonella is not just about microbes conspiring in the shadows. It is about us—our vulnerability, our interconnectedness with unseen worlds, and the ongoing challenge to illuminate the hidden rules that govern life within.
More information: Kanchan Jaswal et al, Commensal yeast promotes Salmonella Typhimurium virulence, Nature (2025). DOI: 10.1038/s41586-025-09415-y