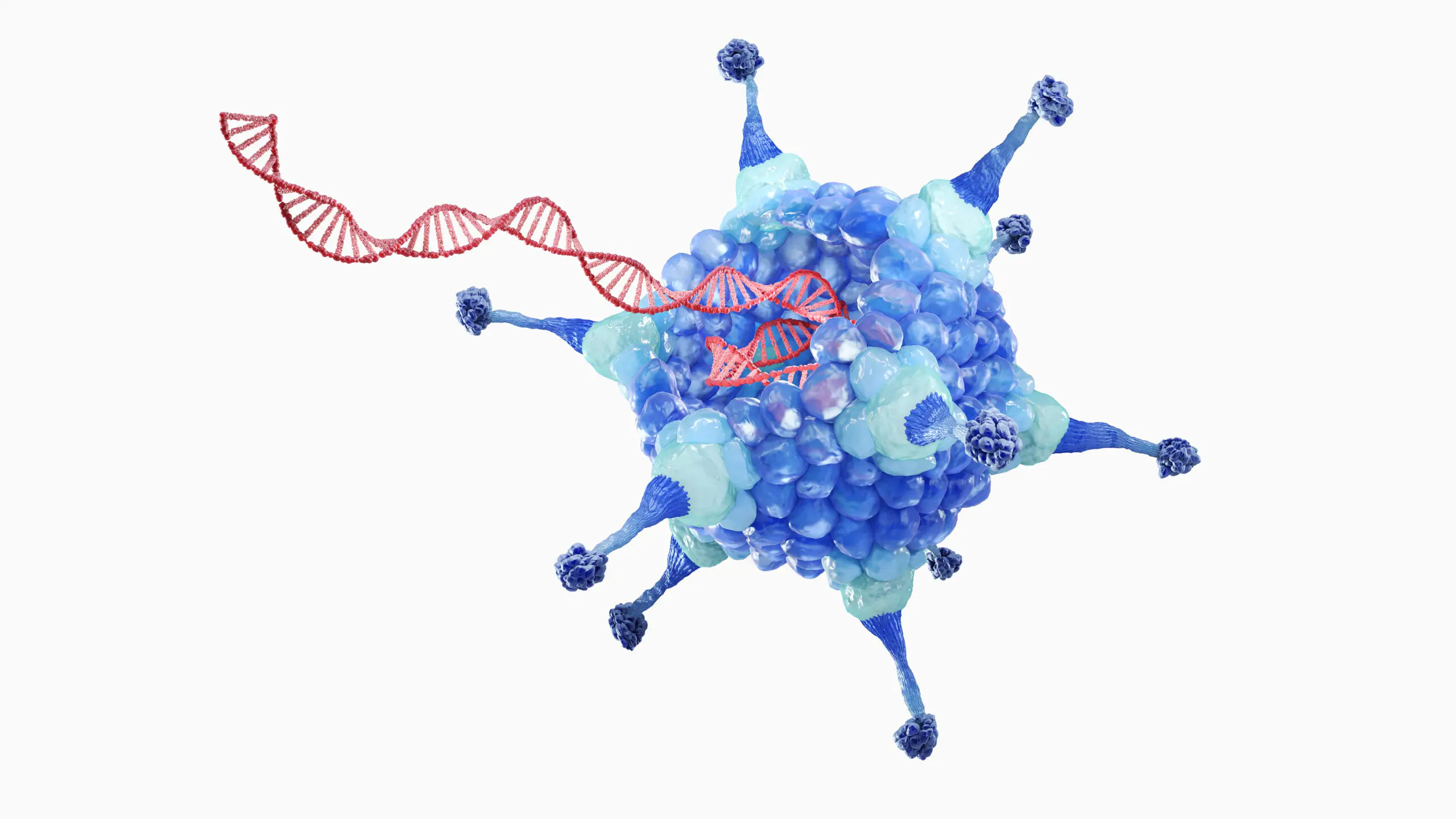
Every cell in your body is a miracle of biology — an intricate symphony of processes, all coordinated by a single language: DNA. It’s our blueprint, the genetic code that determines everything from your eye color to your predisposition to certain diseases. But as with any blueprint, sometimes errors are made. And when those errors occur in our genes, they can lead to devastating consequences — rare genetic disorders, cancers, and diseases once thought untreatable.
For generations, the best medicine could offer was symptom management. If you had a genetic disease, you lived with it — perhaps with drugs, surgeries, or therapies to help along the way. But the root cause — the faulty gene itself — was untouchable.
Until now.
Welcome to the age of gene therapy: the revolutionary field of medicine that doesn’t just treat disease — it aims to correct the very DNA that causes it. Imagine a future where a single injection could silence a deadly gene, replace it with a healthy copy, or even rewrite your genome to eradicate disease altogether.
That future is no longer science fiction. It’s happening now.
The Science of the Genome
To understand gene therapy, you first need a solid grasp of genetics. DNA (deoxyribonucleic acid) is the molecule that carries the genetic instructions for life. These instructions are encoded in sequences of four chemical bases — adenine (A), thymine (T), cytosine (C), and guanine (G). These bases are arranged in specific patterns that form genes, each responsible for a particular protein or function in the body.
There are approximately 20,000–25,000 genes in the human genome. Some genes determine basic traits like height or blood type, while others are involved in complex processes like immune response, brain development, or metabolism.
Sometimes, mutations occur — changes in the DNA sequence that disrupt a gene’s function. These mutations can be inherited, occur spontaneously, or result from environmental damage (such as radiation or chemicals). A mutation in a critical gene can cause genetic disorders like:
- Cystic fibrosis (a lung and digestive disorder)
- Duchenne muscular dystrophy (progressive muscle degeneration)
- Sickle cell anemia (a blood disorder)
- Hemophilia (a clotting disorder)
- Huntington’s disease (a neurodegenerative condition)
Traditionally, treatments aimed to manage symptoms, not fix the gene itself. But what if we could go to the source — the DNA — and make it right?
That’s the promise of gene therapy.
What Is Gene Therapy?
Gene therapy is a biomedical technique that uses genetic material — DNA or RNA — to treat, prevent, or potentially cure disease. It works by directly altering the genetic instructions within a person’s cells.
There are several strategies gene therapy can use:
- Replacing a faulty gene with a healthy copy.
- Inactivating a malfunctioning gene that’s causing problems.
- Introducing a new or modified gene that helps the body fight disease.
In practice, this involves inserting new genetic material into a patient’s cells — usually via a carrier called a vector, most commonly a modified virus engineered to deliver the gene safely without causing illness.
Once inside the cell, the new gene integrates into the genome (or functions separately) and begins producing the correct protein that the defective gene failed to make. In effect, it reprograms the cell to function normally.
Viral Vectors: Turning Viruses into Cures
Ironically, some of our greatest allies in gene therapy are viruses — the very agents we usually associate with disease. Why? Because viruses are nature’s most efficient genetic delivery systems.
Viruses work by inserting their own genetic material into a host cell. Gene therapy harnesses this mechanism by removing the virus’s harmful genes and replacing them with therapeutic DNA.
The most commonly used viral vectors include:
- Adeno-associated virus (AAV): Small, stable, and less likely to provoke immune responses. Widely used in clinical trials.
- Lentivirus: Derived from HIV, these can integrate into the host’s genome and are useful for long-term expression.
- Retrovirus: Similar to lentivirus, but less stable. Earlier gene therapy efforts often used retroviruses.
- Adenovirus: Good at delivering genes to non-dividing cells but can provoke strong immune responses.
Each vector has its pros and cons — and the choice depends on the disease being targeted, the type of cells involved, and the therapeutic goal.
Delivery Methods: Getting Genes Where They Need to Go
There are two main methods for delivering gene therapy:
1. In vivo (inside the body): The gene is delivered directly into the patient’s body, often via an injection into the bloodstream, muscle, or affected organ. This approach is used when the target cells are hard to remove, like neurons or cardiac cells.
2. Ex vivo (outside the body): The patient’s cells are removed, genetically modified in a lab, and then reinserted into the body. This is commonly used in blood disorders, where stem cells can be taken from bone marrow, edited, and returned.
Each approach has its benefits. In vivo is less invasive, while ex vivo offers more control and safety because the edited cells can be tested before being reintroduced.
Curing Genetic Diseases: Real-Life Success Stories
While gene therapy is still in its early stages, several groundbreaking treatments have already made it to clinical use — and changed lives.
Sickle Cell Disease and Beta-Thalassemia:
These are genetic blood disorders caused by mutations in the hemoglobin gene. In 2023, the FDA approved two gene therapies — Casgevy (using CRISPR-Cas9) and Lyfgenia — which modify stem cells to produce functional hemoglobin. Patients have gone from frequent hospitalizations to being virtually symptom-free.
Spinal Muscular Atrophy (SMA):
A deadly disease in infants caused by a missing SMN1 gene. The gene therapy Zolgensma delivers a working copy of the SMN1 gene via an AAV vector. A single intravenous dose can transform a child’s prognosis from near-certain death to near-normal development.
Leber’s Congenital Amaurosis (LCA):
This rare genetic eye disorder causes blindness. The gene therapy Luxturna introduces a healthy copy of the RPE65 gene directly into the retina, restoring vision in children and adults.
Severe Combined Immunodeficiency (SCID):
Sometimes called “bubble boy disease,” SCID prevents the body from fighting infections. Gene therapy has successfully cured this condition by inserting functional immune genes into the patient’s bone marrow cells.
Each of these treatments represents more than science — it represents hope. People who once faced death or lifelong disability now have a future.
CRISPR: The Scissors That Edit Life
No discussion of gene therapy is complete without mentioning CRISPR-Cas9, the revolutionary gene-editing technology that allows scientists to cut and paste DNA with unprecedented precision.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) originated from bacteria’s natural immune system. Scientists adapted this mechanism to create a molecular tool that can:
- Target specific DNA sequences
- Cut them precisely
- Insert, delete, or replace genetic material
CRISPR is fast, cheap, accurate, and versatile. It’s already being used in clinical trials for blood disorders, cancers, and inherited blindness.
More advanced versions — like base editing and prime editing — can now change a single DNA letter without cutting the strand at all, reducing the risk of unintended mutations.
With CRISPR, gene therapy is no longer about delivering a working gene. It’s about repairing the faulty one at its source — a true genetic cure.
The Challenges of Gene Therapy
Gene therapy holds enormous promise, but it also faces serious challenges:
1. Immune Response:
The body can recognize viral vectors as foreign invaders and mount an immune attack. This can reduce treatment effectiveness or even cause dangerous inflammation.
2. Targeting Specific Cells:
Delivering the gene to the right cells, at the right dose, and ensuring it stays active over time is complex. Some tissues are harder to reach than others (e.g., the brain).
3. Insertional Mutagenesis:
If a gene inserts itself randomly into the genome, it could disrupt a vital gene or activate a cancer-causing one. New vectors aim to reduce this risk by targeting safe regions of the genome.
4. Cost:
Gene therapy is expensive. Treatments like Zolgensma and Luxturna cost over $2 million per dose. While one-time cures may be cost-effective in the long run, access remains a huge challenge.
5. Ethical Concerns:
As our ability to edit genes grows, so do ethical dilemmas. Should we edit embryos to prevent disease? What about enhancing traits like intelligence or strength? Where do we draw the line between therapy and enhancement?
Somatic vs. Germline Gene Therapy
Most current gene therapies target somatic cells — the cells in your body that don’t pass genes to offspring. These changes affect only the treated individual.
But germline gene therapy involves editing eggs, sperm, or embryos — changes that are inherited by future generations.
Germline editing is controversial. While it could prevent devastating hereditary diseases, it also raises ethical questions about consent (future generations can’t consent), unintended consequences, and the potential for “designer babies.”
As of now, germline editing is banned in many countries — and hotly debated in the scientific community.
Gene Therapy for Cancer
Beyond rare genetic diseases, gene therapy is making waves in oncology.
CAR-T cell therapy is a groundbreaking technique where a patient’s T-cells are genetically modified to recognize and attack cancer cells. This therapy has led to dramatic remissions in certain types of leukemia and lymphoma.
Researchers are also developing gene therapies that:
- Insert tumor-suppressing genes
- Silence oncogenes (cancer-promoting genes)
- Enhance the immune system’s ability to detect tumors
Gene therapy might not just treat cancer — it might eventually prevent it by editing out cancer-prone genes in high-risk individuals.
The Future: What Comes Next?
The field of gene therapy is evolving rapidly. Here’s what the future may hold:
- Gene therapy for common diseases: Most current therapies target rare disorders. But researchers are working on gene-based treatments for conditions like Alzheimer’s, diabetes, heart disease, and HIV.
- In-body gene editing: Tools like CRISPR will allow doctors to edit genes directly inside the body, without removing cells.
- Personalized medicine: With genetic screening, therapies can be customized to each patient’s unique DNA.
- Nanotechnology: Future gene delivery systems may use nanoparticles instead of viruses, reducing immune reactions.
- Global access: As technology matures, costs may drop, and gene therapy could become accessible worldwide, not just in wealthy nations.
Final Thoughts: Rewriting the Human Story
Gene therapy is more than a new treatment. It’s a shift in how we think about medicine, biology, and even human destiny.
For centuries, we treated symptoms and managed diseases. Now, we’re learning to correct the code of life itself. We can repair, replace, and redesign our own biology — not in some distant future, but today.
It’s easy to get caught up in the hype, but make no mistake: gene therapy is not magic. It’s science, hard-earned and painstakingly developed. It carries risks, challenges, and unknowns. But its potential is nothing short of revolutionary.
We stand at the dawn of a new era — one where disease might not just be fought, but prevented. One where genetic destiny is not written in stone, but open to revision. One where the next generation might inherit not just our genes, but the power to fix them.
In the story of humanity, gene therapy might be the chapter where we stopped being prisoners of biology — and became its authors.