Prostate cancer has long been a quiet specter, shadowing the lives of millions of men around the world. In its early stages, it whispers through symptoms or, more often, lies silently in wait, detectable only through routine screening. For many, the disease is caught early and brought to heel with hormone therapies or surgery. But for others—up to 20% of advanced cases—something more sinister begins to take shape.
It’s a transformation as chilling as it is mysterious: prostate cancer that once responded well to treatment suddenly breaks the rules. It mutates, not through random chaos, but by following a precise biological script. The once-tame tumor becomes aggressive, unrecognizable, and untreatable. This is neuroendocrine prostate cancer (NEPC)—an elusive and deadly shape-shifter with no effective cure.
Now, for the first time, scientists have pulled back the curtain on that transformation. In a landmark study published in Nature Genetics, researchers from Emory University have unraveled the step-by-step process by which prostate cancer cells undergo their deadly metamorphosis. Their findings not only explain how the disease evolves, but more importantly, reveal a new way to stop it.
When Cancer Changes Its Face
Led by Dr. Jindan Yu, a professor of urology at the Emory School of Medicine, the research team tackled one of the most stubborn mysteries in oncology: why prostate cancer often returns in a more lethal form, even after successful treatment. “Prostate cancer is one of the most commonly diagnosed cancers, affecting countless patients and families,” Dr. Yu said. “While it often responds well to hormone therapy, many cases eventually develop resistance.”
The reason lies in the cancer’s ability to adapt. Hormone therapies work by blocking androgen receptors—the biological gears that drive tumor growth in most prostate cancers. But over time, some cells evolve. Stripped of their androgen receptors, they don’t die. They adapt. They become something else entirely: neuroendocrine prostate cancer, a variant that doesn’t rely on hormones and is immune to existing treatments.
“NEPC is a new beast,” Yu said. “It lacks the targets for existing prostate cancer drugs. It’s as if we’ve been fighting one enemy, and suddenly, the enemy changes shape and speaks a different language.”
Seeing the Invisible: Mapping the Mutation in 3D
To confront this enemy, Yu and her colleagues had to first understand it. That meant tracing the subtle changes inside the cancer cells—watching, in essence, as the disease rewired itself from the inside out. To do that, they used some of the most advanced genomic tools available today.
In partnership with Dr. Jonathan Zhao, associate professor of human genetics, the team created the first-ever three-dimensional map of how prostate cancer cells reorganize their DNA structure as they shift into NEPC. Unlike flat sequencing data, which provides a list of genetic information, this 3D mapping reveals how the DNA physically folds and loops inside the nucleus. Those loops, it turns out, are not random. They form structures that activate new genes and silence others—essentially rewriting the identity of the cell.
The results were breathtaking. The map showed a deliberate choreography of genetic remodeling—a kind of architectural metamorphosis where the very shape of the genome changed to support the cancer’s new, more aggressive form.
Unlocking the Genetic Gatekeepers
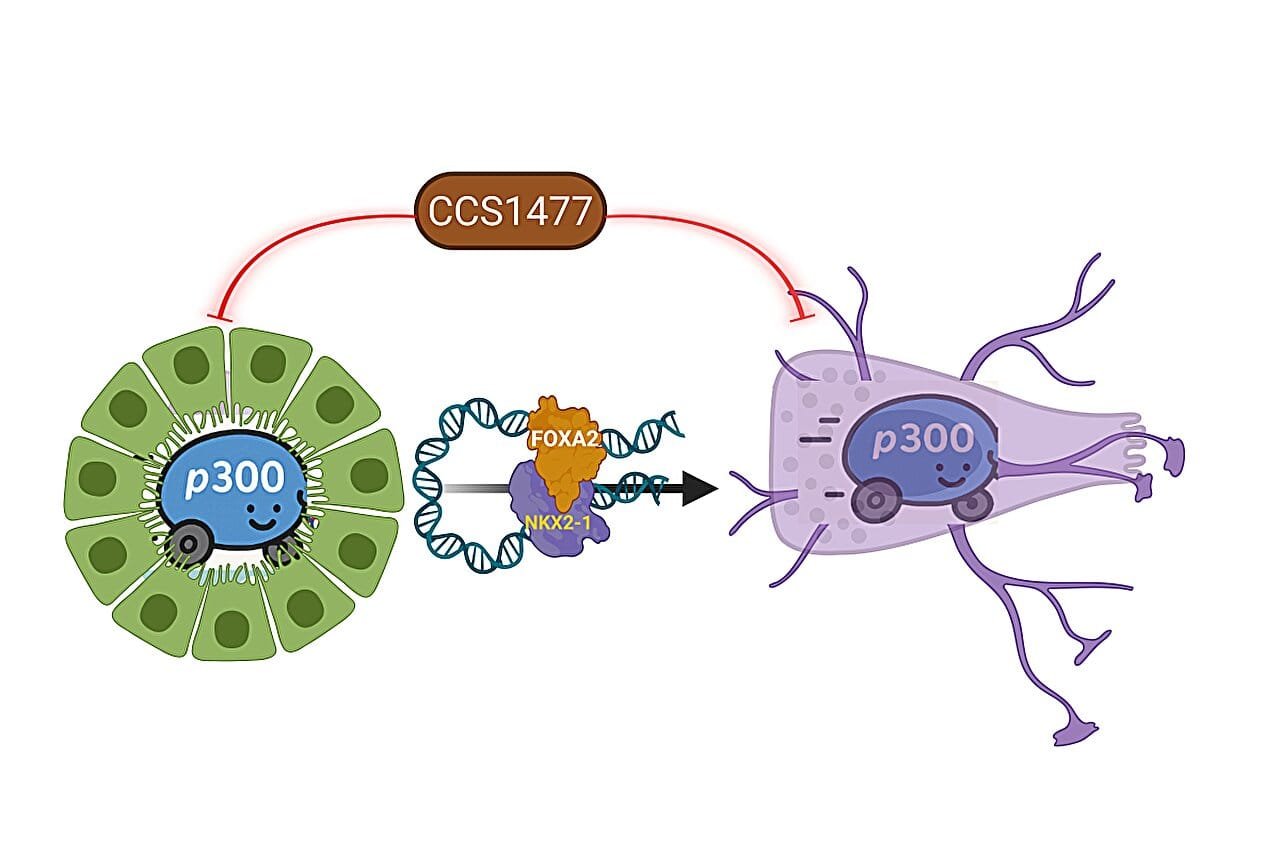
At the center of this transformation were two proteins—FOXA2 and NKX2-1. Like molecular architects, these proteins didn’t just participate in the change—they orchestrated it.
FOXA2, described as a “pioneer factor,” had the remarkable ability to pry open tightly packed regions of DNA, revealing hidden stretches of genetic code. These newly exposed genes were never meant to be expressed in prostate tissue—they were from an entirely different cellular lineage. Once FOXA2 unlocked the door, NKX2-1 entered. This protein, normally active in brain and lung cells, flipped a genetic switch. It began activating instructions that reprogrammed the cell from a prostate tumor to a neuroendocrine one.
Together, these two proteins performed a kind of cellular betrayal. They didn’t just change the behavior of the cancer cells—they changed their very identity. They turned the cells into something alien, something faster, more aggressive, and—most heartbreakingly—resistant to the therapies that once held them in check.
The Molecular Matchstick: CBP and p300
But transformation wasn’t just a matter of gene activation. It required fuel—coactivators that would amplify this new genetic program and lock the changes into place. That’s where another set of players came into view: CBP and p300.
These enzymes act like matchsticks, igniting gene expression by modifying chromatin and enhancing transcription. In the context of NEPC, CBP and p300 essentially turbocharged the reprogramming process. Without them, the transformation stalled.
This discovery was more than academic—it pointed to a weak link in the cancer’s armor. If CBP and p300 were required for the metamorphosis, perhaps disabling them could halt the disease’s progression.
A Ray of Hope: Stopping the Shape-Shifter
Targeting CBP and p300 had been explored in other cancers, but never in the context of neuroendocrine prostate cancer. Yu’s team decided to test a class of drugs already in clinical trials—CBP/p300 inhibitors. One drug in particular, CCS1477, showed remarkable promise.
In lab cultures and animal models, the researchers found that this drug effectively blocked the transformation to NEPC. Not only that, it halted the growth of existing neuroendocrine tumors. The cells, stripped of their molecular drivers, could no longer sustain their deadly new identity.
For families affected by advanced prostate cancer, this discovery opens a new chapter of hope. What was once considered an unstoppable progression may now be interrupted, perhaps even reversed. “We are not just watching cancer evolve anymore,” Yu said. “We are learning how to stop it in its tracks.”
The Larger Implications: Science That Feels Personal
The science behind this discovery is complex, layered with data and dense with molecular jargon. But at its heart, it speaks to something deeply human—the fight against a disease that has touched millions of lives.
Prostate cancer doesn’t just alter the bodies of those who suffer from it. It reshapes families, burdens relationships, and tests the resilience of caregivers and communities. Treatments that once offered hope can turn into long, draining routines. Recurrence feels like betrayal. The shift to NEPC feels like an ambush.
But this new research offers something rare in the fight against cancer: clarity. It shows us how the enemy moves, how it hides, and where it’s vulnerable. And in doing so, it allows the scientific and medical community to respond with precision, purpose, and—perhaps most importantly—empathy.
What Comes Next
While this study is a landmark, it is also a beginning. Clinical trials involving CCS1477 and related compounds are already underway, and the next few years will be critical in determining how these insights translate to patient care.
There is still much to learn. Why do some patients experience this transformation while others don’t? Are there early warning signs? Can resistance be predicted before it happens? Yu’s team and others around the world are now equipped with new tools to answer these questions.
For now, the achievement stands as a testament to what’s possible when curiosity meets compassion—when researchers dare to look deeper, to map what was once invisible, and to believe that understanding leads to healing.
Reference: Xiaodong Lu et al, NKX2-1 drives neuroendocrine transdifferentiation of prostate cancer via epigenetic and 3D chromatin remodeling, Nature Genetics (2025). DOI: 10.1038/s41588-025-02265-4