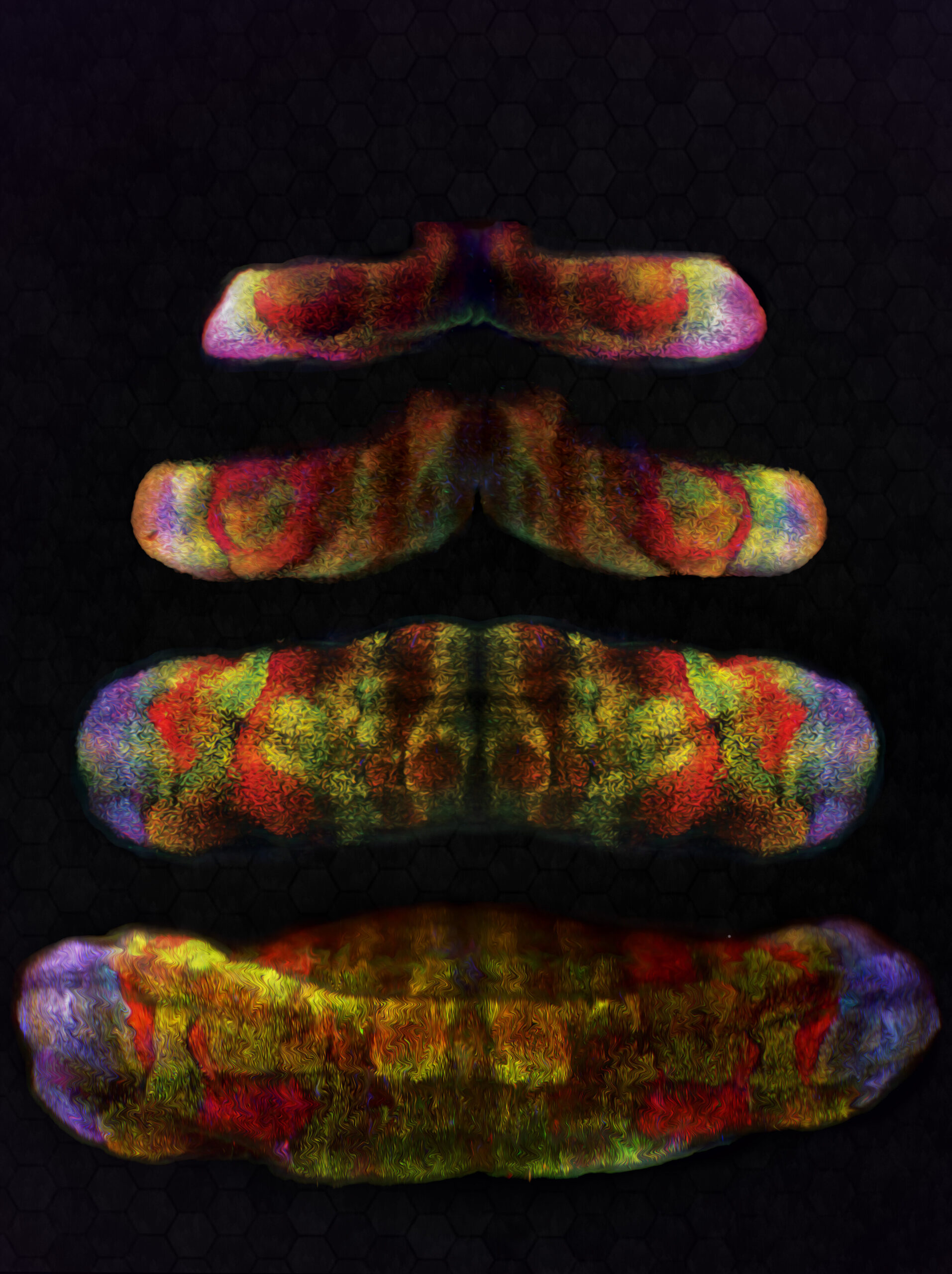
When most people think of the brain, they imagine the wrinkled surface of the cerebral cortex, the region responsible for thought, memory, and consciousness. Yet tucked quietly at the back of the skull lies another structure—the cerebellum, often called the “little brain.” For centuries, the cerebellum was seen as a specialist in movement: the conductor ensuring our muscles work in harmony so that we can walk, run, or dance without stumbling.
But recent discoveries have begun to change this picture. The cerebellum is not just about balance and coordination—it may also play a crucial role in higher mental functions, from speech to complex thinking. At the heart of this revelation are the Purkinje cells, the cerebellum’s remarkable neurons, and a family of genes known as FOXP. Together, they are reshaping how we understand brain development, language, and even the origins of human intelligence.
The Majestic Purkinje Cells
Purkinje cells are giants among neurons. With their sprawling, tree-like branches, they stand out as some of the most visually stunning cells in the nervous system. These cells act as the cerebellum’s gatekeepers: they integrate massive amounts of incoming information and then relay signals outward, influencing everything from motor control to higher brain functions.
For a long time, scientists believed Purkinje cells were relatively uniform, serving similar roles across the cerebellum. But new research has revealed a more nuanced truth: Purkinje cells are not all the same. They exist in multiple subtypes, each tuned to perform specialized functions in different regions of the cerebellum. This diversity may hold the key to understanding how the cerebellum contributes not just to movement, but also to complex abilities such as speech, learning, and abstract thought.
FOXP Genes: The Molecular Switchmasters
The story of Purkinje cells cannot be told without introducing the FOXP genes. This family of four genes—FOXP1, FOXP2, FOXP3, and FOXP4—are transcription factors. In simple terms, they act like master switches, turning other genes on or off, orchestrating the molecular symphony that shapes how cells develop and function.
Two members of this family, FOXP1 and FOXP2, are especially intriguing. They are strongly linked to human development and communication. Mutations in FOXP2 can lead to profound speech and language difficulties, while FOXP1 mutations are associated with delayed development, intellectual disability, and traits resembling autism. FOXP2 is so fundamental to vocalization that it is shared across species: it supports song learning in birds, echolocation in bats, pup calls in mice, and, in humans, the miracle of speech.
Until recently, however, the exact role of these genes in shaping the cerebellum—and Purkinje cells in particular—was largely a mystery.
A New Window into the Cerebellum
A team of researchers at the University of Connecticut School of Medicine, led by James Y.H. Li, set out to fill this gap. Their goal was bold: to map the genetic and neural processes shaping Purkinje cell diversity, and to uncover how FOXP genes guide cerebellar development.
To do this, the team combined cutting-edge techniques. First, they used single-cell RNA sequencing (scRNA-seq) to profile gene activity in thousands of individual Purkinje cells from developing mouse embryos. This allowed them to detect subtle molecular differences between cells that would otherwise look identical under a microscope.
But sequencing alone could not reveal where these cells were located within the cerebellum. To solve this, the researchers developed a powerful new method called scANKRS (single-cell Anchoring Network of Key Regulators to Space). This tool let them map the molecular fingerprints of Purkinje cell subtypes back onto their physical locations in brain tissue. To add another layer, they used 3D light-sheet fluorescence imaging, which provided vivid, spatially detailed views of gene expression across the cerebellum.
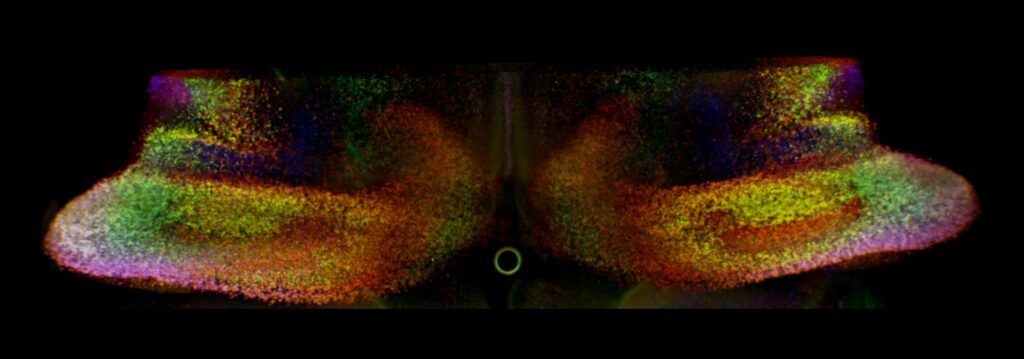
Together, these techniques painted the most detailed picture yet of how Purkinje cells diversify—and how FOXP genes influence their fate.
Eleven Shades of Purkinje Cells
The findings were striking. The researchers identified at least 11 distinct Purkinje cell subtypes, each defined by unique molecular signatures and localized to specific regions of the cerebellum. This diversity suggests that different clusters of Purkinje cells specialize in different tasks, helping the cerebellum handle both motor coordination and higher-order mental functions.
Crucially, the FOXP1 and FOXP2 genes played central roles in this diversification. When the scientists selectively deleted these genes in mice, the cerebellum’s architecture changed dramatically. Not only were Purkinje cells affected, but the entire process of forming cerebellar hemispheres—the paired structures linked to advanced motor and cognitive skills—was disrupted. This is the first genetic evidence directly tying FOXP genes to hemisphere formation.
A Surprising Link to Vocalization
Perhaps the most fascinating finding came when the researchers looked at behavior. Mice lacking FOXP1 or FOXP2 in their cerebellum struggled to produce vocalizations. For animals that rely on calls to communicate—whether pups crying for their mothers or adults signaling to one another—this is a profound impairment.
The implication is clear: the cerebellum, long thought to be focused on movement, is deeply involved in vocalization and perhaps speech. Purkinje cell diversity, shaped by FOXP genes, may provide the neural foundation for the sophisticated communication that defines humanity.
Evolutionary Clues
The research also offers tantalizing hints about brain evolution. Li and colleagues observed that Foxp1-positive Purkinje cells—a subtype heavily influenced by FOXP1—are abundant in the human fetal cerebellum but rare in birds. This suggests that the expansion of these specific Purkinje cells may have been critical in the evolutionary leap that allowed mammals, and especially humans, to develop advanced cerebellar hemispheres. These structures are strongly associated with higher cognitive functions such as problem-solving, planning, and language.
In other words, our ability to speak, reason, and create may be rooted, at least in part, in the molecular evolution of FOXP genes and the specialization of Purkinje cells.
Clinical Implications
Beyond evolutionary insights, this work carries important medical relevance. Disorders such as autism spectrum disorder (ASD), language impairments, and intellectual disabilities have been linked to FOXP1 and FOXP2 mutations. By showing how these genes shape cerebellar development and Purkinje cell diversity, the research provides new pathways for understanding—and potentially treating—these conditions.
If future studies confirm these findings in primates and humans, therapies aimed at modulating FOXP activity or supporting specific Purkinje cell subtypes could open the door to new treatments for developmental disorders that affect speech, language, and cognition.
The Road Ahead
Li and his team are far from finished. Their next steps involve mapping the molecular mechanisms of FOXP transcription factors in even greater detail: identifying their binding partners, their downstream target genes, and the regulatory networks that orchestrate cerebellar growth. They also plan to test how manipulating individual Purkinje cell subtypes influences cerebellar hemisphere expansion and function.
This work is not only unraveling one of neuroscience’s great mysteries but also providing a framework for understanding how tiny genetic switches shape the grand architecture of the brain.
A New Vision of the “Little Brain”
The cerebellum may be smaller than the cerebral cortex, but it is proving to be no less important. Far from being a simple motor control center, it emerges as a hub where movement, speech, and higher cognition converge. Purkinje cells, sculpted by the guiding hand of FOXP genes, give the cerebellum its versatility and power.
The story of the cerebellum reminds us that the brain is never what we assume. Just when we think we understand its divisions—movement here, thought there, emotion somewhere else—it surprises us with hidden connections. And within those connections may lie the deepest secrets of what makes us human.
More information: Nagham Khouri-Farah et al, FOXP genes regulate Purkinje cell diversity and cerebellar morphogenesis, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02042-w. www.nature.com/articles/s41593-025-02042-w