Schizophrenia, a condition that affects how a person thinks, feels, and perceives reality, has long stood as one of medicine’s most enigmatic challenges. Now, an international team of scientists led by Cardiff University’s Centre for Neuropsychiatric Genetics and Genomics (CNGG) has made a major breakthrough.
In the largest exome-sequencing study of schizophrenia to date, researchers have identified eight genes that play a role in the disorder — offering fresh insight into the biological roots of a condition that affects around 24 million people worldwide.
The study, published in Nature Communications under the title “Whole-exome sequencing analysis identifies risk genes for schizophrenia”, marks a pivotal step toward unraveling the intricate genetic code behind schizophrenia and opens new pathways for developing future treatments.
The Largest Study of Its Kind
To understand what makes this discovery so important, it helps to consider the scale of the work. The researchers analyzed genetic data from 28,898 people diagnosed with schizophrenia, compared with 103,041 individuals without the condition, as well as 3,444 families affected by the disorder.
By focusing on rare, high-impact mutations in protein-coding genes — the “instruction manuals” for making proteins in our cells — the team was able to pinpoint variations that were more common in individuals with schizophrenia. These subtle differences, buried deep within DNA, can alter how brain cells develop, communicate, and function.
Two Genes With Strong Links, Six More With Growing Evidence
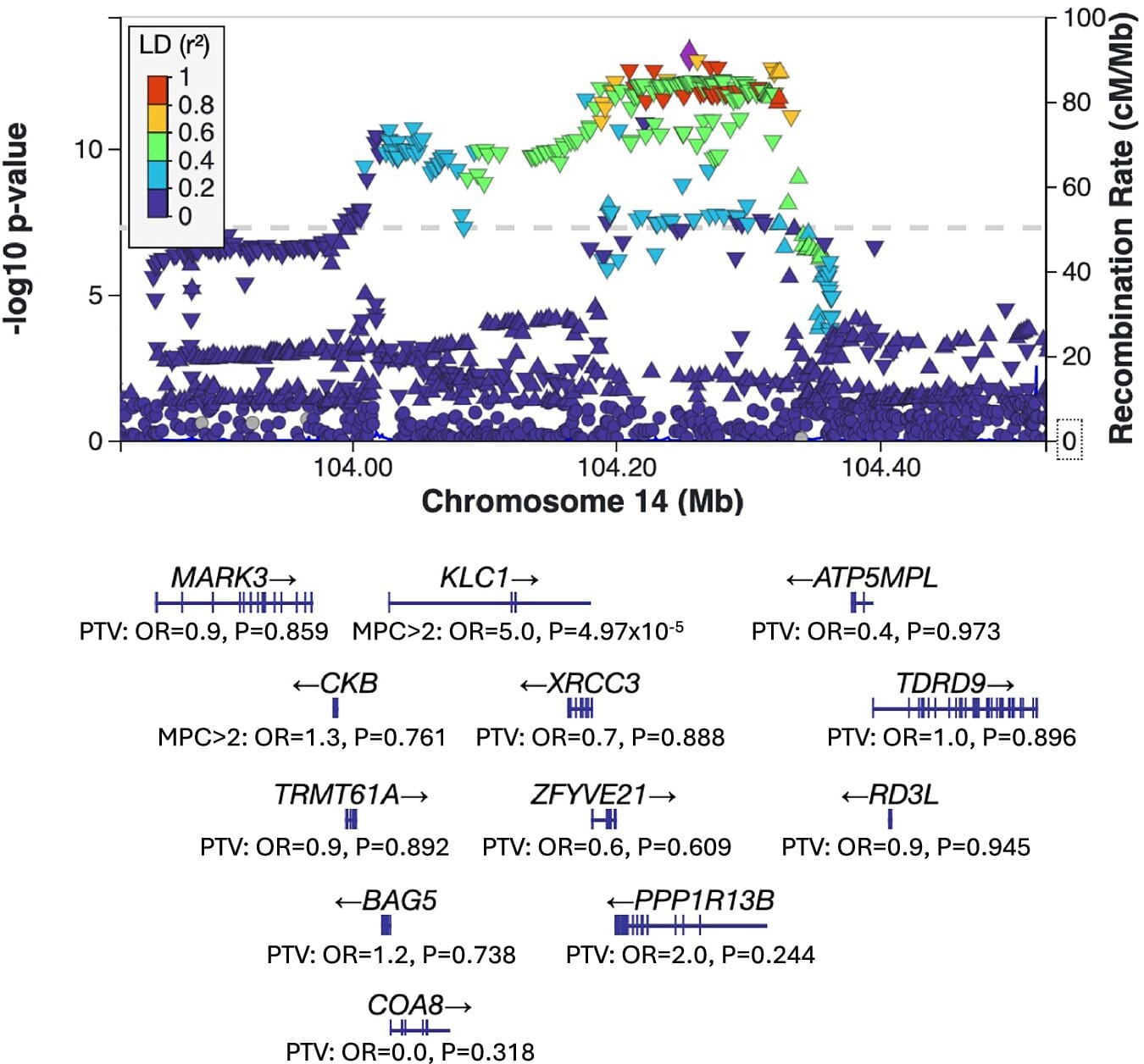
The study revealed two genes with particularly strong ties to schizophrenia: STAG1 and ZNF136. Both play crucial roles in cellular processes, with STAG1 especially notable for its involvement in how DNA is packaged and regulated inside cells.
Six other genes were also identified as potential players in schizophrenia: SLC6A1, KLC1, PCLO, ZMYND11, BSCL2, and CGREF. While the evidence for these was more moderate, the findings provide compelling new directions for future investigation.
Notably, SLC6A1 and KLC1 stand out as the first schizophrenia risk genes ever linked specifically to missense variants — genetic alterations that change the amino-acid sequence of proteins, effectively tweaking their structure and potentially their function. This discovery sharpens scientists’ understanding of how tiny genetic changes may ripple outward to affect brain chemistry.
Insights Into Brain Communication and DNA Organization
The findings don’t just expand the list of schizophrenia risk genes; they also highlight biological systems that may be central to the disorder.
Ph.D. student Sophie Chick, one of the study’s researchers at Cardiff University, explained:
“These findings are informative because they suggest that schizophrenia might be linked to changes in how DNA is organized within cells, and also disruptions in how brain cells communicate using a chemical called GABA.”
GABA (gamma-aminobutyric acid) is a neurotransmitter that helps balance electrical activity in the brain. If this delicate balance is disrupted, it can profoundly affect perception, thought, and behavior. The genetic links uncovered in this study provide some of the clearest evidence yet that changes in GABA signaling could underlie aspects of schizophrenia.
A Shared Genetic Landscape With Other Disorders
One striking outcome of the study is its reinforcement of the idea that schizophrenia shares genetic roots with other neurodevelopmental conditions. Four of the newly identified genes — STAG1, SLC6A1, ZMYND11, and CGREF1 — have previously been associated with autism, epilepsy, and developmental delay.
This overlap suggests that conditions we often view as separate may, at the genetic level, have interconnected pathways. Understanding these shared mechanisms could accelerate discoveries not only for schizophrenia but also for a range of related brain disorders.
A Blueprint for Future Treatments
Dr. Elliott Rees, lead author of the study from Cardiff University’s School of Medicine, emphasized the importance of the results:
“Rare genetic variants have long been known to have a role in schizophrenia, but identifying specific genes linked to these mutations has been a major challenge. Our findings represent an important step forward by expanding the number of genes now confidently associated with rare variants in the disorder.”
While the research is not an immediate cure, it represents a blueprint for future studies. By mapping out which genes are involved and how they influence brain development and communication, scientists can begin to design more targeted therapies and guide the search for new drugs.
A Step Closer to Hope
Until now, only a handful of genes had been reliably linked to schizophrenia, leaving vast gaps in understanding. This study significantly expands that list and provides a clearer biological foundation for one of psychiatry’s greatest puzzles.
Translating genetic discoveries into effective treatments will take time, but each breakthrough moves the scientific community closer to that goal. For patients and families affected by schizophrenia, these findings offer not only new scientific knowledge but also a growing sense of hope: that the mysteries of the mind are not impenetrable, and that science is steadily unlocking the path to better care.
More information: Sophie L. Chick et al, Whole-exome sequencing analysis identifies risk genes for schizophrenia, Nature Communications (2025). DOI: 10.1038/s41467-025-62429-y