Sometimes the biggest discoveries hide in the smallest details. Researchers at Children’s Hospital of Philadelphia (CHOP) have uncovered that minute fragments of genetic code — called microexons — are missing in some of the most aggressive childhood brain tumors. Their absence doesn’t just alter how tumor cells behave; it may also open a door to new, highly precise immunotherapies that could one day give children a fighting chance against cancers long considered unbeatable.
The findings, recently published in Cell Reports, shine a spotlight on pediatric high-grade gliomas — devastating tumors that strike children and resist nearly every standard treatment.
The Challenge of Treating Brain Cancer
For decades, oncologists have dreamed of harnessing the immune system to destroy cancer. CAR T cell therapy, which engineers immune cells to recognize and kill cancer cells, has transformed treatment for certain blood cancers. But brain tumors present a harsher challenge.
“Blood cancers are one thing,” explained senior author Andrei Thomas-Tikhonenko, Ph.D., of CHOP. “In the brain, you cannot afford collateral damage. If immunotherapy kills healthy neurons alongside the tumor, the consequences are devastating.”
That’s the central dilemma: most tumor cells look too much like healthy brain cells on their surface. Identifying what makes tumor cells unique is essential to safely target them.
The Secret World of Splicing
Every gene in the human body can produce multiple versions of proteins through a process called alternative splicing. Think of it like editing film: the same footage can be cut into different versions of a movie, depending on which scenes are kept or removed.
In brain cells, splicing tends to be particularly intricate, often involving very short genetic segments known as microexons. These tiny fragments, just a handful of nucleotides long, can profoundly influence how proteins fold and function.
Scientists suspected that glioma cells might splice their RNA differently than healthy brain cells. But previous RNA sequencing studies often overlooked microexons, dismissing them as too short to matter.
What the Tumors Were Hiding
By zooming in more carefully, CHOP researchers discovered something startling: pediatric high-grade gliomas consistently skipped specific microexons in genes that code for important surface proteins.
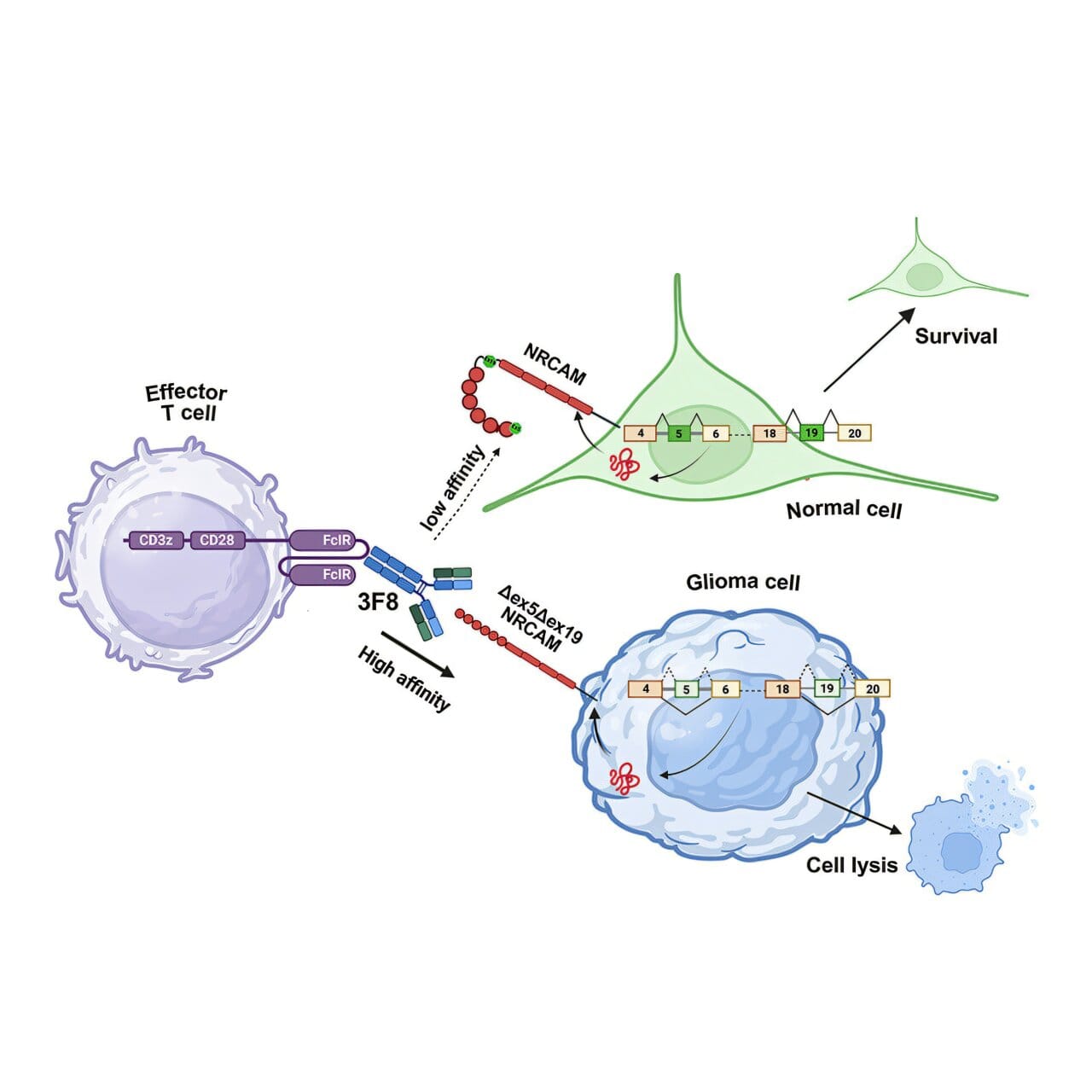
One key example involved NRCAM, a protein that helps neurons stick together and form synapses. In healthy brain cells, NRCAM includes two microexons that allow the protein to fold into its normal shape. But in gliomas, those microexons were skipped. The result was a shortened, altered version of NRCAM with a different 3D structure — a mutant form the tumor relied on for survival and spread.
“When we looked at how tumor cells behaved, this altered NRCAM wasn’t just different — it was essential for their ability to migrate and invade,” said first author Priyanka Sehgal, Ph.D., a research scientist at CHOP. “In both lab dishes and mouse models, tumors depended on it.”
Turning a Weakness Into a Target
The discovery that glioma cells produce a unique version of NRCAM created a tantalizing opportunity: if immunotherapies could recognize the glioma-specific protein but ignore the normal one, it might finally allow doctors to attack tumors without harming healthy neurons.
To test this idea, the team developed a monoclonal antibody that binds only to the glioma-specific NRCAM. When mixed with glioma cells, the antibody acted like a spotlight, tagging cancer cells so that immune cells could find and destroy them.
“While microexons may be small, the effects they have on the overall protein structure are quite profound,” said Thomas-Tikhonenko. “That structural change gave us a handle to design a highly selective antibody.”
From there, the researchers went a step further: they armed T cells with a receptor that recognizes the antibody, creating a system akin to CAR T cells but tuned specifically to the glioma protein. In preclinical experiments, this strategy successfully marked and killed tumor cells.
More Than Just One Tumor
What makes this work especially exciting is its broader potential. The same kind of microexon skipping seen in pediatric gliomas has also been reported in glioblastoma multiforme, one of the deadliest brain cancers in adults, as well as in tumors of neuroendocrine origin.
That means NRCAM — or other splicing-derived targets like it — could represent an entirely new class of “Achilles heels” for solid tumors, a frontier where immunotherapy has struggled to gain traction.
“This could change the way we think about finding targets,” Sehgal explained. “Instead of looking only at mutations in DNA, we’re asking how tumors splice RNA differently. That opens up a whole new map of vulnerabilities.”
From Discovery to the Clinic
Of course, there’s a long journey ahead. The findings remain at the preclinical stage, tested only in laboratory and mouse models. The CHOP team is now working to refine their approach, adapting the antibody-based recognition into CAR T cells and other immunotherapeutic designs that could be safely tested in humans.
Clinical trials will require not only careful engineering but also rigorous testing to ensure safety, given the delicate environment of the brain. Still, the potential is enormous.
A Glimmer of Hope
For families facing a diagnosis of pediatric high-grade glioma, treatment options today are heartbreakingly limited. Survival rates remain low, and even the most aggressive surgeries, radiation, and chemotherapies often cannot stop the disease.
This new research doesn’t yet promise a cure — but it does offer a path forward that wasn’t visible before. By looking where others overlooked — in the smallest slices of RNA — scientists may have uncovered a powerful tool to help the immune system tell friend from foe in the brain.
And that, for children and families living in the shadow of these tumors, is a reason to hope.
More information: Priyanka Sehgal et al, NRCAM variant defined by microexon skipping is a targetable cell surface proteoform in high-grade gliomas, Cell Reports (2025). DOI: 10.1016/j.celrep.2025.116099