For most of us, a broken bone means weeks in a cast and a slow but steady recovery. But when a fracture is severe — after a devastating car crash, a fall from a height, or a battlefield explosion — the body’s natural healing process often breaks down. In “open fractures,” where shattered bone pierces the skin and soft tissue is badly damaged, recovery is uncertain, sometimes impossible. Even with surgery, bone grafts, or metal implants, patients may face permanent disability.
But now, researchers at the Perelman School of Medicine at the University of Pennsylvania, in collaboration with Emory University, have uncovered a surprising ally in the body’s repair arsenal: a special kind of muscle stem cell that can transform into bone. Their findings, published in the Proceedings of the National Academy of Sciences (PNAS), suggest a new way to help bones regenerate — not by replacing them artificially, but by teaching the body to heal itself more effectively.
“This approach takes the lessons we learned and gives the body the boost it needs to naturally heal itself in a much more efficient and effective way,” said senior author Ling Qin, Ph.D., a professor of Orthopedic Surgery at Penn Medicine.
A Discovery Hidden in Plain Sight
For decades, scientists assumed that most broken bones were repaired by stem cells in the periosteum, the thin membrane wrapping every bone. These periosteal stem cells could generate new bone cells, knitting fractures together. But doctors have long observed that periosteum-driven healing is inconsistent, especially in catastrophic injuries where large sections of muscle and skin are lost. Something was missing from the story.
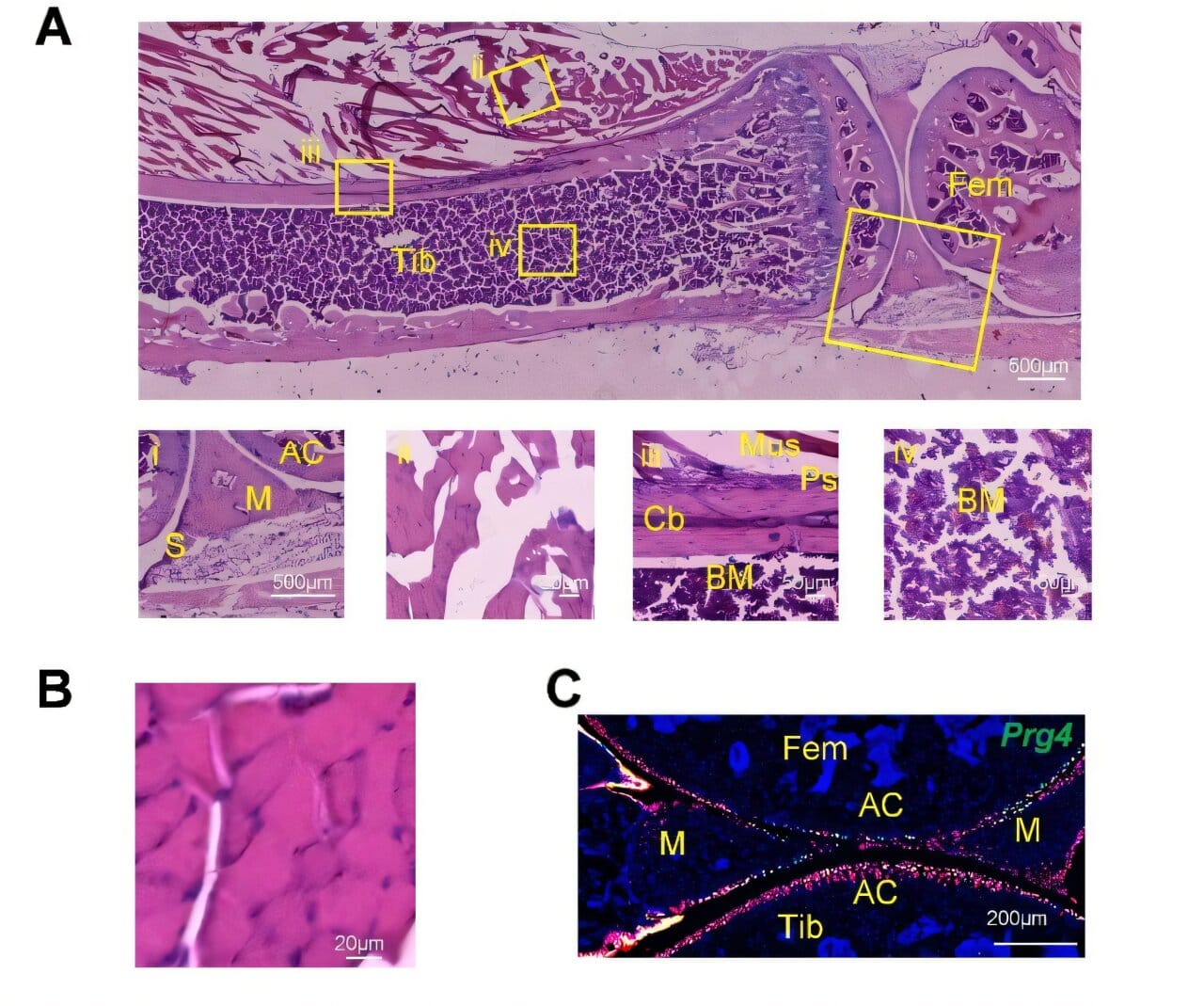
Qin and her colleagues found the missing piece not in the bones themselves but in the muscles that support them. In their experiments with mice, they identified a population of stem cells known as Prg4+ cells — a subtype of fibro-adipogenic progenitors (FAPs), already known to play a role in muscle biology. To their astonishment, these muscle-derived stem cells didn’t just support bone repair indirectly. They could actually transform into bone cells.
This was the first clear evidence that muscle stem cells can cross the boundary between tissues, moving from their home in muscle to take on the identity of bone.
How Muscle Cells Become Bone
In the animal studies, Prg4+ cells acted like first responders at the scene of an accident. When a fracture occurred, they quickly left the muscle and migrated to the injury site. Once there, they began producing the full suite of cell types needed for bone repair: chondrocytes (cartilage cells), osteoblasts (cells that build bone), and osteocytes (mature bone cells that maintain tissue strength).
The process wasn’t just temporary scaffolding. When researchers later examined healed bones, they discovered that the once-muscle-based cells had permanently become bone. Even more remarkably, some of these transformed cells lingered behind as a new pool of periosteal stem cells, essentially preparing the bone for the next time injury might strike.
The team confirmed their importance with a decisive experiment: when Prg4+ cells were deliberately destroyed, fracture healing slowed dramatically. Without them, the repair process faltered.
A New Way to Think About Fractures
The implications are profound. Current treatments focus primarily on bone itself — stabilizing fractures, grafting bone tissue, or stimulating periosteal cells. But Qin’s research suggests that muscle tissue next to bones may be just as critical. Muscles, often thought of only as the engine for movement, may also serve as hidden reservoirs of healing potential.
“Muscles are often overlooked in bone healing,” explained co-author Jaimo Ahn, Ph.D., a professor of Orthopedics at Emory University and a former Penn researcher. “But this work shows that they hold powerful keys to recovery.”
In catastrophic open fractures, where both bone and muscle are destroyed, this finding could open doors to new therapies. But even in more routine injuries, the discovery has promise. Areas with less muscle coverage, such as ankles and knees, may benefit from targeted stimulation of Prg4+ cells. Older adults, whose muscles naturally shrink with age, may also see improved healing if these stem cells can be activated.
From Discovery to Treatment
So how could this science move from the lab to the clinic? The researchers see two possible paths. One is to design growth factor–based drugs or small molecules that stimulate Prg4+ cells already present in a patient’s muscles, coaxing them into action after injury. The other is to develop therapies where activated Prg4+ cells are introduced directly into fractures, seeding the site with ready-made repair workers.
Both approaches could dramatically shorten recovery times and improve outcomes for patients who today face months of immobility or incomplete healing.
Qin is already planning the next steps: “We want to dive deeper into how other fibro-adipogenic progenitor stem cells behave and whether they hold similar repair abilities. This could be a whole new chapter in regenerative medicine.”
Healing Bones by Harnessing Nature
The discovery of Prg4+ cells as muscle-to-bone transformers reshapes our understanding of how the body heals itself. It shows that healing is not limited to a single tissue or organ but is a cooperative act, with muscles stepping in to rescue bones when their own repair systems fall short.
For patients, the hope is that one day, the devastation of a crushed leg after a car crash, or the lingering frailty after a hip fracture in old age, may no longer mean a lifetime of disability. Instead, with a boost from our own hidden stem cells, the body could rebuild itself stronger, faster, and more completely than ever before.
And it all began with a discovery hiding in plain sight — that sometimes, muscles don’t just move bones. They can become them.
More information: Qi He et al, Prg4 + fibroadipogenic progenitors in muscle are crucial for bone fracture repair, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2417806122