Imagine drifting uncontrollably into sleep in the middle of a conversation, a meeting, or while crossing a busy street. Now imagine that, just as unpredictably, your muscles give way—your knees buckle, your arms slump, and you collapse to the floor, wide awake but physically paralyzed. For individuals living with narcolepsy type 1, this is a daily and disorienting reality.
Narcolepsy type 1 is a chronic neurological disorder that disrupts the brain’s ability to regulate sleep-wake cycles. Its defining features—excessive daytime sleepiness (EDS) and sudden bouts of muscle weakness known as cataplexy—are not merely nuisances but profound disruptions to safety, productivity, and quality of life. At the heart of this condition lies the near-total absence of a key neuropeptide: orexin, also known as hypocretin.
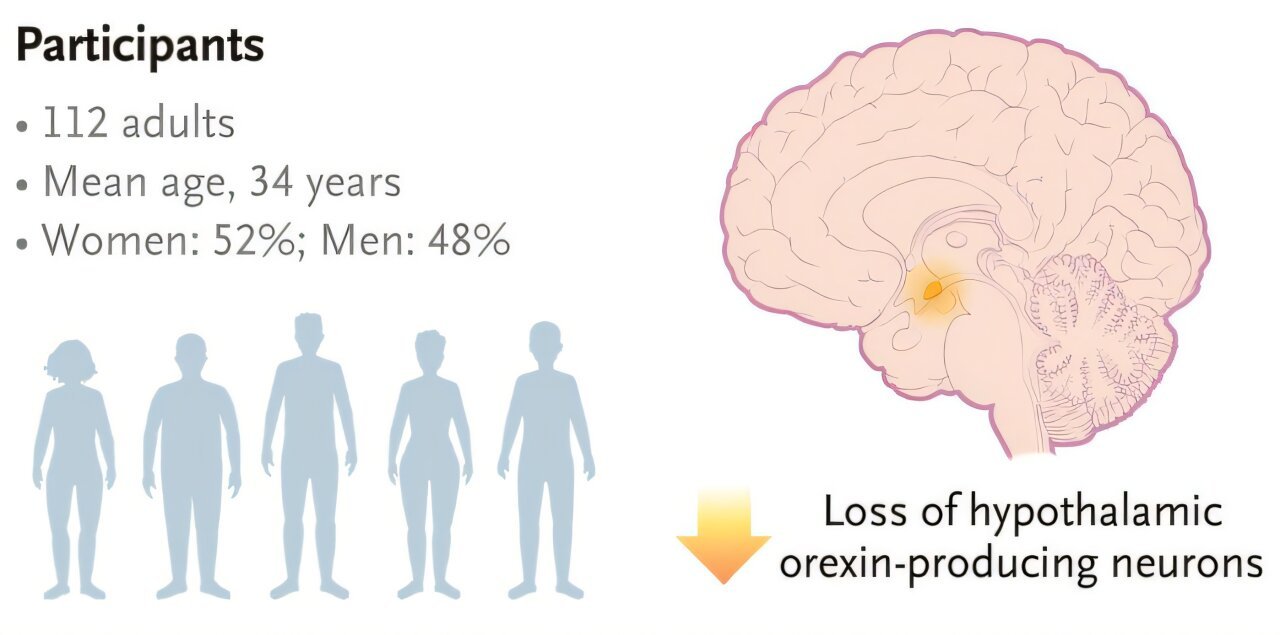
Orexin plays a vital role in stabilizing wakefulness and preventing premature transitions into REM sleep, the stage associated with dreaming and muscle atonia. In people with narcolepsy type 1, the immune system mistakenly destroys the orexin-producing neurons deep in the hypothalamus, leaving the brain vulnerable to erratic sleep transitions. The result: an unrelenting tug-of-war between consciousness and unconsciousness.
The Promise and Perils of Orexin-Based Therapies
For decades, narcolepsy treatments have focused on managing symptoms. Stimulants like modafinil and amphetamine-based drugs promote wakefulness, while antidepressants and sodium oxybate help control cataplexy. But none of these target the root of the problem—the missing orexin signal. And most come with substantial drawbacks: dependency risks, cardiovascular effects, and burdensome dosing schedules.
The dream of directly replacing or mimicking orexin signaling became a scientific North Star. In recent years, orexin receptor 2 (OX2R) agonists emerged as promising candidates to replicate the natural wake-promoting function of orexin. By selectively activating OX2R, these compounds aim to restore alertness and stabilize REM sleep transitions without triggering the anxiety or euphoria often linked to stimulant-based drugs.
But early attempts came with a catch: hepatotoxicity. Some of the most promising OX2R agonists produced concerning elevations in liver enzymes, halting clinical progress and raising serious safety questions.
Now, a new molecule may be changing that narrative.
Enter Oveporexton: A Precise Awakening
In a collaborative effort led by the Gui de Chauliac Hospital in Montpellier, France, and the University of Bologna in Italy, researchers have identified a novel compound that may finally deliver on the promise of orexin-based therapy without the specter of liver damage: oveporexton.
Described in the New England Journal of Medicine, the team conducted a Phase II, double-blind, randomized, placebo-controlled trial to evaluate oveporexton’s efficacy and safety in individuals diagnosed with narcolepsy type 1. This isn’t just another line in the long list of sleep aids—oveporexton is a selective oral OX2R agonist, engineered to mimic orexin’s effects with surgical precision.
What sets oveporexton apart is not just what it does, but what it avoids: namely, the liver-related toxicity that has plagued earlier orexin-targeting drugs.
Designing the Trial: A Global Inquiry into Wakefulness
The study enrolled 112 adult participants between the ages of 18 and 70, hailing from international sites across Europe, the United States, and Japan. Participants were randomized via sophisticated interactive response technology, ensuring a robust allocation to one of five treatment groups: four active dosing regimens of oveporexton and one placebo group.
The drug was administered orally over eight weeks, with dosages ranging from 0.5 mg twice daily to a complex regimen of 2 mg followed by 5 mg once daily. Sleep and wakefulness metrics were rigorously tracked using a battery of standardized tests:
- The Maintenance of Wakefulness Test (MWT) evaluated participants’ ability to resist sleep under ideal sleep-inducing conditions.
- The Epworth Sleepiness Scale (ESS) offered a self-reported measure of daytime drowsiness.
- Participants kept detailed cataplexy diaries, logging every episode of muscle weakness they experienced.
The data collected painted a vivid picture—one that suggests oveporexton could soon revolutionize how we treat this enigmatic condition.
From Slumber to Strength: What the Numbers Reveal
The results were striking.
In the MWT, participants receiving oveporexton demonstrated marked improvements in the time they could remain awake. Average sleep latency increased by 12.5 to 25.4 minutes, depending on the dosing group. In contrast, those on placebo actually lost ground, with a 1.2-minute decrease in wakefulness.
Meanwhile, ESS scores—where higher values indicate greater sleepiness—plummeted by 8.9 to 13.8 points among oveporexton recipients. Those on placebo saw only a modest 2.5-point reduction.
But the most eye-opening findings emerged from the cataplexy frequency data. Participants receiving placebo reported an average of 8.76 cataplexy episodes per week. In comparison:
- The 0.5 mg twice-daily group averaged 4.24 episodes,
- The 2 mg twice-daily group had 3.14 episodes, and
- The 2 mg followed by 5 mg daily group saw a reduction to just 2.48 episodes per week.
Only the higher-dosed groups met the bar for statistical significance, underscoring a dose-dependent effect in cataplexy control. The ability to reduce muscle-weakening episodes by over 70% in some participants marks a major therapeutic breakthrough.
Side Effects and Safety: The Other Side of the Coin
No drug is without side effects, and oveporexton was no exception. The most common adverse events reported were insomnia (48%), urinary urgency (33%), and urinary frequency (32%). Yet importantly, none of these side effects led to treatment discontinuation.
Moreover, liver enzyme levels remained stable across the dosing groups—a critical win for a class of drugs previously marred by hepatotoxicity.
Participants also showed improvement on the Narcolepsy Severity Scale for Clinical Trials (NSS-CT) and in overall quality-of-life metrics, particularly the 36-Item Short Form Health Survey (SF-36). These are more than just numbers; they suggest that patients felt not only more awake, but more human—more themselves.
Beyond the Numbers: A New Paradigm for Treatment
The implications of this study ripple far beyond its eight-week span. If future trials replicate these findings, oveporexton could represent the first safe, long-term pharmacologic mimic of the missing orexin signal in narcolepsy type 1. Unlike existing treatments, which either stimulate the central nervous system indiscriminately or suppress REM sleep through indirect mechanisms, oveporexton offers targeted neural repair.
It’s a pharmacological stand-in for what the brain has lost—a chemical prosthetic that restores not just wakefulness, but the integrity of sleep architecture.
What’s more, the oral formulation makes it vastly more convenient than sodium oxybate, which requires nighttime dosing and strict regulation due to its abuse potential. For the narcoleptic community, oveporexton could mean freedom: from crashes, from cataplexy, and from the constant dread of losing control of one’s own consciousness.
What Comes Next?
The path to approval is still unfolding. Phase III clinical trials and long-term extension studies are now underway, aiming to test oveporexton’s durability and safety in larger, more diverse populations. These studies will also investigate whether chronic dosing sustains benefits, and whether tolerance or unforeseen side effects emerge over time.
Still, the findings thus far have ignited cautious optimism among sleep researchers. If the early data hold up, oveporexton could join a short list of transformative medications—like insulin for diabetes or levodopa for Parkinson’s—that address not just symptoms, but the underlying biochemical deficit.
The Future of Wakefulness: An Orexin Renaissance
The development of oveporexton also signals a broader renaissance in orexin biology. Beyond narcolepsy, researchers are exploring the role of orexin signaling in depression, addiction, and even Alzheimer’s disease. As we deepen our understanding of this powerful neuropeptide system, drugs like oveporexton may become the first of many tools in a new kind of neurological toolkit.
But for now, narcolepsy patients stand to gain the most. After years of inadequate options and medications that offered only partial relief, oveporexton offers something rare in the field of neurological disorders: hope.
Hope for a life less interrupted. Hope for steady feet beneath them and clear skies above. Hope for waking up—not just from sleep, but into a life that feels whole again.
Reference: Yves Dauvilliers et al, Oveporexton, an Oral Orexin Receptor 2–Selective Agonist, in Narcolepsy Type 1, New England Journal of Medicine (2025). DOI: 10.1056/NEJMoa2405847