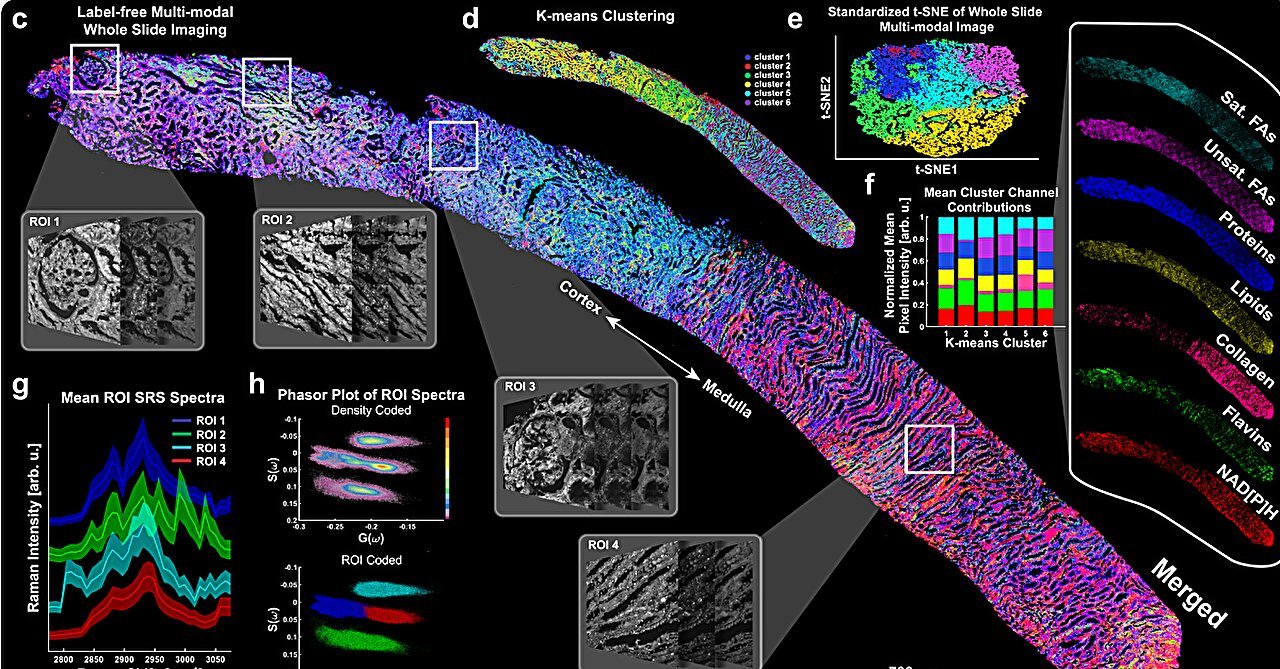
In the quiet pulse of the human body, the kidneys act as unseen sentinels—purifying blood, balancing fluids, and orchestrating the silent symphony of internal chemistry. But when these essential organs begin to falter, the damage can be insidious, often surfacing only after the harm has taken deep root. Diabetic kidney disease (DKD), for instance, is one of the world’s leading causes of kidney failure, yet detecting it early remains a significant clinical challenge.
Now, an innovative team of scientists from the University of California San Diego and Washington University School of Medicine in St. Louis has unveiled a breakthrough technology that could change how we see and understand kidney disease—literally.
Published in Nature Communications, their pioneering work introduces a label-free multimodal optical biopsy system that allows researchers to peer inside kidney tissue in exquisite, three-dimensional detail—without the need for slicing, staining, or damaging the sample.
Imagine a camera that can see not just shapes and shadows, but the biochemical whispers of disease, captured in light and color. That’s the promise of this new imaging system: to deliver a non-destructive, AI-enhanced view into the kidneys’ structural and molecular world—and possibly to transform how kidney disease is diagnosed and managed.
Seeing the Invisible: The Science Behind Label-Free Imaging
Traditional kidney biopsies are workhorses of diagnostic medicine, but they come with trade-offs. A tissue sample is typically sliced into ultra-thin sections, stained with dyes, and examined under a microscope. Each step introduces artifacts, consumes precious material, and can miss subtle—but critical—biological changes.
The new optical biopsy technique sidesteps all of that.
Led by Dr. Lingyan Shi, a bioengineering professor at UC San Diego, and Dr. Sanjay Jain, a physician-scientist at Washington University in St. Louis, the team designed a label-free multimodal imaging system that uses lasers to map tissue in three dimensions, layer by layer, at microscopic resolution. This isn’t your standard microscope. It’s a multi-physics marvel that taps into a trio of advanced optical techniques:
- Two-photon fluorescence (TPF)—to detect natural autofluorescent molecules like NADH and FAD that report on cellular metabolism.
- Second harmonic generation (SHG)—to reveal structural proteins like collagen, flagging signs of fibrosis or scarring.
- Stimulated Raman scattering (SRS)—to identify chemical signatures of fats, proteins, and lipids—important metabolic markers in kidney disease.
Together, these methods illuminate a biochemical atlas of the kidney, all without touching the tissue with a single dye or knife.
“It’s like giving clinicians X-ray vision,” says Shi. “Except instead of just seeing bones, we can see cells, metabolites, and collagen fibers—live and intact.”
Why This Matters: Catching Disease in Its Infancy
The kidneys are deceptively complex. Each one contains roughly a million nephrons—microscopic filtering units composed of blood vessels, tubules, and support cells that work in concert to cleanse the blood. In diabetic kidney disease, this harmonious structure begins to fray: capillaries leak, tubules thicken, and fibrotic scars silently spread.
Yet by the time traditional imaging detects these changes, damage is often extensive and irreversible.
That’s where label-free optical biopsy shines. In the new study, researchers applied their system to kidney samples from both healthy mice and diabetic models. What they found was startling: early-stage metabolic and structural changes invisible to standard techniques were rendered clearly in the label-free images.
Fats accumulating where they shouldn’t. Collagen forming ghostly threads of scar tissue. Changes in protein distribution hinting at cellular stress.
“We’re seeing the adaptations and maladaptations happening at the cellular level,” explains Jain. “These are the earliest footprints of disease, and the earlier we can detect them, the better chance we have to intervene and preserve kidney function.”
Built for the Clinic: Fast, Clean, and AI-Ready
Speed and simplicity are at the heart of this platform’s clinical potential.
Because it doesn’t rely on staining, sample prep time is dramatically reduced. And since the method is non-destructive, the tissue remains available for further molecular testing—an important advantage when biopsy material is scarce or precious.
But what really pushes this technology toward clinical readiness is its integration with artificial intelligence.
Using machine learning algorithms, the team trained models to detect and classify pathological features in the imaging data automatically. That includes quantifying fat deposits, identifying fibrotic areas, and recognizing telltale changes in metabolic markers—all in a matter of minutes.
“We’re creating a pipeline that can go from tissue to insight quickly,” says Dr. Tony Fung, the paper’s first author and a former doctoral student in Shi’s lab, now at Yale University. “That opens up possibilities not only for research but for point-of-care diagnostics.”
In the future, the system could be used during kidney biopsies to provide near-instant feedback—helping physicians make decisions about diagnosis, treatment, or the need for further testing in real time.
Beyond the Kidney: A Platform for Disease Discovery
Though this study focuses on diabetic kidney disease, the potential applications stretch far beyond nephrology.
“Anywhere tissue structure and metabolism are altered, this tool could be informative,” says Shi. That includes liver diseases, neurological disorders, cancer, and even cardiovascular conditions. The multimodal approach—blending metabolic and structural imaging—gives scientists a new lens to examine diseases as complex interactions between molecules, cells, and tissue architecture.
In essence, the label-free biopsy system represents a paradigm shift: a way to watch disease unfold without disturbing the very tissue being studied.
It’s not just about diagnosis—it’s about understanding. How does fibrosis begin? Why do certain cells accumulate fat under diabetic stress? Which molecular changes come first in disease progression? These are questions that the system may help answer, not in decades, but now.
Building the Future of Biomedical Imaging
Developing the label-free imaging system was a feat of interdisciplinary collaboration. It required innovations in optics, software, biology, and machine learning—a convergence that speaks to the future of biomedical engineering.
Fung, during his time in Shi’s lab, not only built the hardware but also designed the data analysis pipeline and AI framework. His work is a model for the next generation of researchers who must wear many hats—engineer, coder, biologist, and data scientist.
“This is the future of imaging,” says Jain. “Not just clearer pictures, but smarter ones—data-rich, multidimensional, and context-aware.”
The researchers are now working to miniaturize the system and explore ways to make it compatible with live tissue imaging. The goal is a compact, user-friendly device that could be integrated into clinical workflows—bringing high-resolution, AI-enhanced imaging from the lab bench to the bedside.
A Gentle Revolution in Medicine
For all its technological sophistication, what makes this breakthrough profound is its gentleness. In a medical world often defined by invasive tests and aggressive interventions, the label-free multimodal optical biopsy offers a different approach—one that listens to tissue rather than distorting it.
It’s a quiet revolution, but one with deep implications.
Because beneath the surface of our cells and organs lies a symphony of changes—chemical, structural, metabolic—that precede disease by months or even years. Capturing those changes early, accurately, and non-destructively could be the key to transforming how we treat not only kidney disease but a wide range of chronic conditions.
As Einstein once said, “Look deep into nature, and then you will understand everything better.” With this new technology, scientists are doing exactly that—looking deeper, seeing more, and reshaping the future of diagnosis.
And perhaps, just in time, offering new hope to millions living in the shadow of kidney disease.
Reference: Anthony A. Fung et al, Label-free multimodal optical biopsy reveals biomolecular and morphological features of diabetic kidney tissue in 2D and 3D, Nature Communications (2025). DOI: 10.1038/s41467-025-59163-w