Imagine a child with autism flapping their hands for hours, or a person with obsessive-compulsive disorder washing their hands dozens of times a day. These repetitive behaviors, though seemingly simple, often mask a complex storm brewing in the brain—one that science has long struggled to decode. Now, a groundbreaking study led by Professor Jiwon Um at the Daegu Gyeongbuk Institute of Science and Technology (DGIST) may have finally illuminated one of the brain’s most elusive enigmas.
Published in the prestigious journal Cell Reports, the research doesn’t just hint at a link—it maps out a complete biological pathway connecting chronic brain inflammation to meaningless repetitive behaviors. The implications are profound. With this discovery, scientists may be able to target and treat the root cause of certain symptoms in autism spectrum disorder (ASD) and obsessive-compulsive disorder (OCD), not just manage them.
Beyond the Behavior: The Neuroscience of Repetition
Repetitive behaviors are not inherently pathological. From twirling hair to tapping fingers, they can offer comfort, focus, or simply serve as harmless quirks. But in neurodevelopmental and psychiatric conditions like ASD and OCD, such behaviors can become exaggerated, compulsive, and disruptive. Until now, the exact neural mechanics driving these behaviors remained an open question.
Previous research has pointed to abnormal neural circuits and genetic mutations as contributing factors, and indeed, both ASD and OCD are highly heritable. However, there was a missing link—something that could explain how these genetic and neurological predispositions translate into the visible, often distressing, behaviors seen in patients.
Professor Um’s team may have found that link in an unexpected place: the immune cells of the brain.
Microglia, the Brain’s Silent Watchmen
At the heart of this discovery are microglia, the brain’s resident immune cells. Traditionally viewed as janitors, cleaning up debris and damaged neurons, microglia have recently gained attention as dynamic regulators of brain health and disease. When functioning properly, they keep the brain’s environment balanced. But when chronically activated—like in neuroinflammatory conditions—they can become problematic.
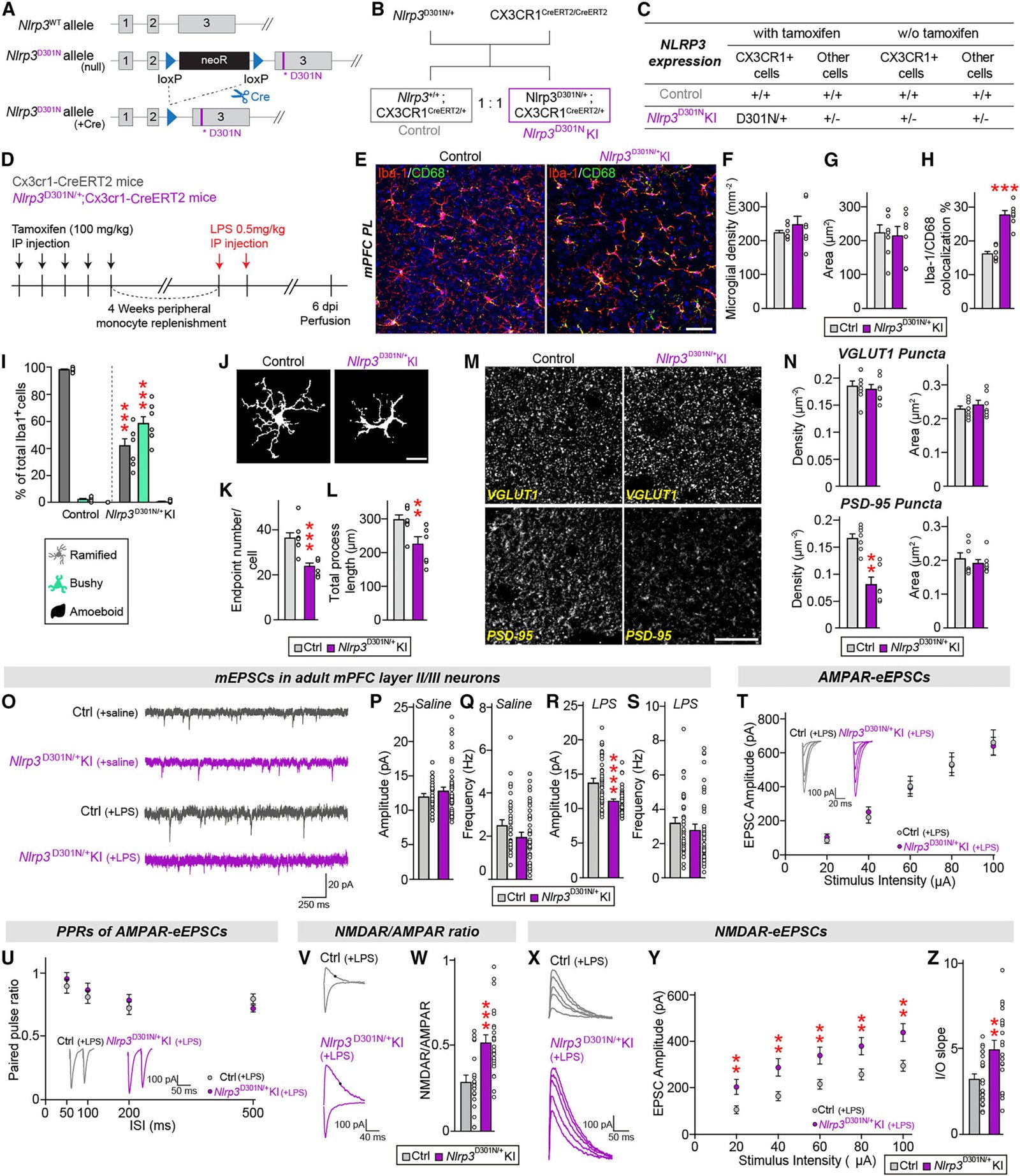
The researchers focused on a specific genetic mutation in mice: a malfunction in the NLRP3 gene. This gene is part of a family responsible for regulating inflammation. Mutations in NLRP3 lead to persistent microglial activation, creating a state of chronic brain inflammation.
This persistent inflammatory state, the study found, had striking consequences. It triggered behavioral abnormalities in mice that mirrored the compulsive, meaningless repetition seen in human ASD and OCD. These weren’t just quirks of behavior—they were the visible symptoms of an immune system gone awry inside the brain.
Glutamate Gone Wild: The Role of NMDA Receptors
To understand how inflammation leads to repetitive behaviors, we have to look at the chemical messengers that allow brain cells to communicate. Chief among them is glutamate, the most abundant excitatory neurotransmitter in the brain. Think of it as the accelerator in the neural system—it gets neurons firing, sparks thought, fuels learning.
Glutamate works through several types of receptors, and among the most critical are NMDA (N-methyl-D-aspartate) receptors. These receptors act like volume knobs on the cell membrane: too low, and communication is sluggish; too high, and the circuit overloads.
The study revealed that chronic inflammation—specifically, the kind sparked by hyperactive microglia—caused a dangerous overactivation of NMDA receptors. This “glutamate storm” effectively short-circuited the brain’s control systems, giving rise to repetitive behaviors in the affected mice.
In simple terms: chronic brain inflammation rewired the mice’s brains into loops of compulsive action.
From Insight to Intervention: Testing Memantine
Once the researchers identified the NMDA receptor overactivity as the behavioral trigger, they asked a simple yet powerful question: What if we could tone it down?
They turned to memantine, a drug already approved by the U.S. Food and Drug Administration (FDA) to treat Alzheimer’s disease. Memantine works by selectively blocking overactive NMDA receptors, preventing excitotoxicity without completely silencing essential brain activity.
When administered to mice carrying the NLRP3 mutation, memantine had a striking effect. Repetitive behaviors dropped dramatically. The drug essentially restored balance to the NMDA receptors, which, in turn, normalized the animals’ behavior.
This is a landmark finding—not just because of what it reveals about brain biology, but because it offers a shortcut to treatment. Rather than spending years developing and testing new drugs, we may be able to repurpose existing, safe, FDA-approved medications to help individuals suffering from ASD or OCD.
The Inflammatory Messenger: Interleukin-1 Beta
But how, exactly, does chronic inflammation flip the NMDA receptors into overdrive?
Here’s where the research gets even more granular. The inflamed microglia weren’t just sitting there glowing with irritation—they were actively releasing a potent inflammatory molecule called interleukin-1 beta (IL-1β). This cytokine, already implicated in a wide range of autoimmune and inflammatory diseases, turned out to be a key player in modulating glutamate signaling.
IL-1β binds to its receptors in the brain and effectively turbocharges NMDA receptor activity. More IL-1β means more NMDA signaling, which means more repetitive behavior. This chain of influence gave the team another therapeutic target: block IL-1β’s action, and you might break the cycle.
So they tested anakinra, another FDA-approved drug, which inhibits the interleukin-1 receptor. Originally developed for rheumatoid arthritis, anakinra crosses the blood-brain barrier and can calm inflammation at its source. The results were stunning—mice treated with anakinra showed normalized NMDA activity and, more importantly, a disappearance of repetitive behaviors.
Implications for Autism and OCD Treatment
What makes this research truly revolutionary is the potential for immediate clinical impact. Memantine and anakinra are already on the market. Both have been tested extensively for safety and are well-tolerated in diverse patient populations. This opens the door for rapid human trials to test their efficacy in ASD and OCD.
Professor Um’s team has essentially laid out a molecular roadmap: from inflammation (via NLRP3 mutation), to microglial activation, to IL-1β release, to NMDA receptor overactivity, and finally to behavioral manifestation. At each step in the chain, there’s an opportunity for therapeutic intervention.
And because these drugs already exist, we’re not starting from scratch. This is the power of drug repurposing—taking medications developed for one disease and applying them to another. In this case, Alzheimer’s and arthritis medications could help unlock better treatments for neurodevelopmental and psychiatric disorders that have resisted conventional therapies.
The Future of Neuroimmune Psychiatry
The broader implication of this work is that inflammation may be a common denominator in many psychiatric and neurodevelopmental conditions. For decades, psychiatry focused almost exclusively on neurotransmitters—dopamine, serotonin, glutamate—without considering how immune signals might influence brain function.
But now, thanks to studies like this, a new field is emerging: neuroimmune psychiatry. It recognizes that the immune system and the nervous system are deeply intertwined. When one falters, the other suffers.
This study not only identifies a novel mechanism underlying repetitive behaviors but also reframes our understanding of ASD and OCD as potentially inflammatory conditions—at least in part. That means therapies targeting inflammation could be beneficial not just for cognitive and behavioral symptoms, but perhaps even for mood, anxiety, and sensory processing.
A Personal Triumph and a Scientific Turning Point
In reflecting on the work, Professor Um said, “This study has demonstrated that chronic brain inflammation induces overactivity in NMDA glutamate receptors, resulting in repetitive behavioral disorders. We may be able to suggest a new therapeutic approach for treating ASD and OCD, which are often accompanied by repetitive behaviors.”
It’s more than a modest conclusion. It’s a triumph of molecular detective work, of translational neuroscience, and of scientific perseverance. In a landscape where millions of people suffer from repetitive behaviors that interfere with daily life, this research offers something increasingly rare: hope grounded in evidence.
And perhaps most importantly, it reminds us that in the intricate web of human behavior, the immune system and the brain are not strangers. They are intimate partners, each capable of shaping the other in profound, life-altering ways.
Reference: Hyeji Jung et al, The NLRP3 inflammasome in microglia regulates repetitive behavior by modulating NMDA glutamate receptor functions, Cell Reports (2025). DOI: 10.1016/j.celrep.2025.115656