Within the dark, winding corridors of the human intestine, a silent war is always underway. This isn’t the kind of war most people imagine—there are no swords, no roaring battles. Yet the stakes are impossibly high: the integrity of the digestive tract, the absorption of nutrients, and ultimately, our health and survival. On one side stand the invaders—bacteria, viruses, and parasites that find the gut a fertile breeding ground. On the other, our immune system, a vast and intricate defense network, is constantly making decisions: attack or tolerate?
Now, a groundbreaking discovery by researchers at the Research Institute of the McGill University Health Centre (RI-MUHC) has added a profound new chapter to our understanding of this hidden war. Led by Professor Irah King, a team of immunologists, stem cell biologists, and infectious disease experts has revealed a previously unknown mechanism by which the immune system defends the gut—not by attacking the invaders directly, but by protecting the tissue itself from the consequences of persistent infection.
Published in Cell, one of the world’s most prestigious scientific journals, the study reveals a surprising role for interferons—molecules typically associated with the fight against viruses and bacteria—in shielding the structure of the intestine during long-term infection with helminths, or parasitic worms. This mechanism, which maintains gut function even when parasites persist, could pave the way for revolutionary treatments for intestinal infections and chronic inflammatory diseases alike.
The Puzzle of the Peaceful Parasite
Helminths are ancient parasites that have co-evolved with humans for thousands of years. More than two billion people globally—especially in areas with poor sanitation—will experience a helminth infection at some point in their lives. Yet these worms often manage to live in the human intestine for months, sometimes years, without causing overt disease.
This paradox fascinated Professor King, Senior Scientist in the Translational Research in Respiratory Diseases Program at the Institute. “One way our immune system protects us is by destroying viruses or bacteria,” King explains. “However, some pathogens, such as helminths, have found ways to avoid being killed. They can remain in the intestine without triggering disease. Why doesn’t our immune system eliminate them—or why doesn’t their presence cause more damage?”
The team’s curiosity led them into uncharted territory. Rather than focusing on how the immune system kills parasites, they decided to explore how it manages to live with them, maintaining intestinal health even in their persistent presence.
Interferons: From Pathogen Killers to Tissue Guardians
Interferons are well-known in immunology circles as first responders to viral invasion. They are signaling proteins—molecular messengers that alert cells to the presence of infection and stimulate a cascade of defensive responses. But no one expected them to be key players in responding to helminths, which are not viruses at all, but large multicellular organisms.
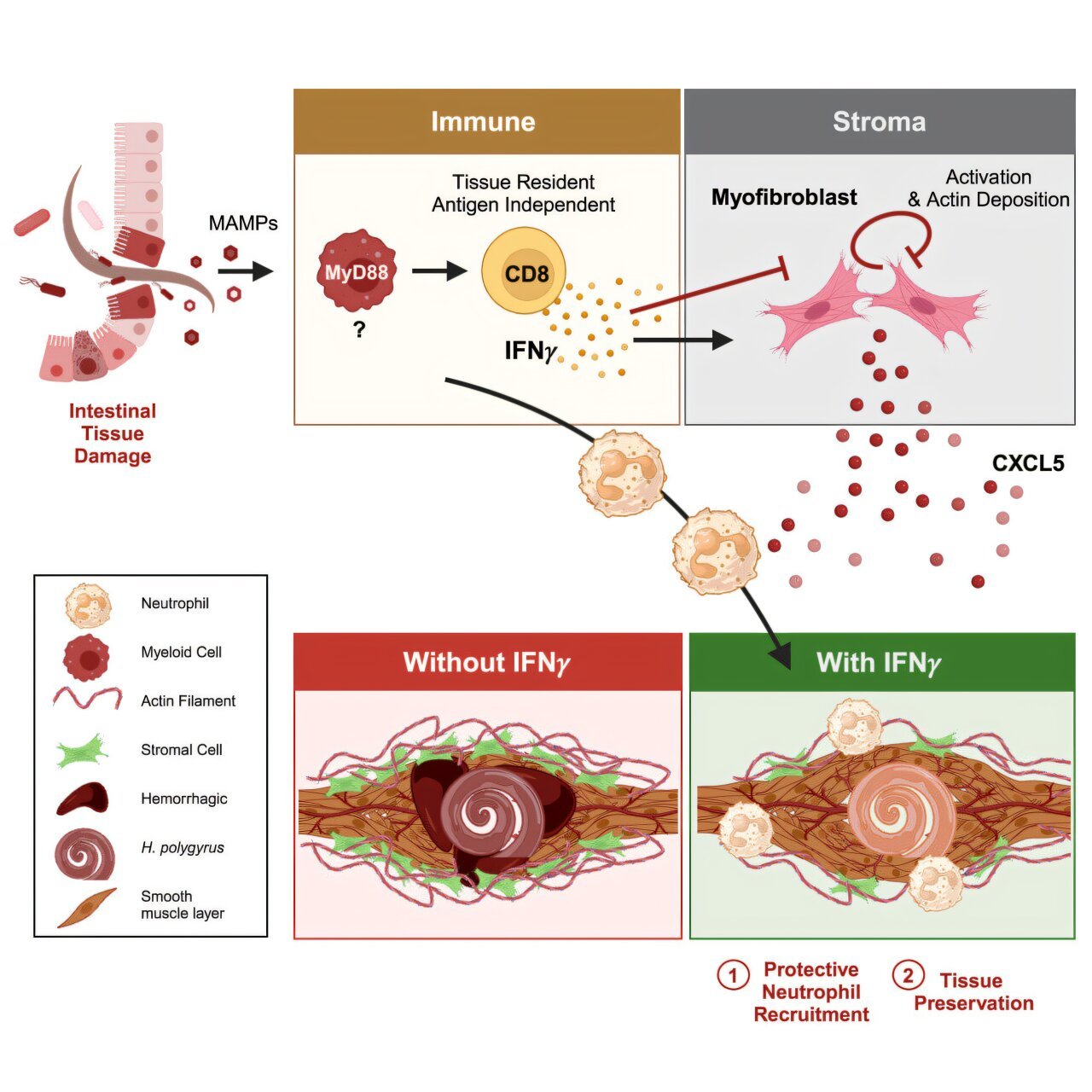
When the team infected mice with intestinal helminths and tracked immune responses, they were surprised to see a spike in interferon activity. Even more surprisingly, these interferons had no measurable effect on the worms themselves. The parasites remained largely unbothered.
So what, then, were these interferons doing?
Using genetic tools to block interferon signaling specifically in the intestinal stroma—the structural cells that form the backbone of the organ—the researchers uncovered something remarkable. Without interferon signals reaching the stroma, the intestine suffered dramatic damage during helminth infection. The mice experienced intestinal bleeding and profound functional impairment. The immune system, it seemed, wasn’t just tolerating the infection—it was actively maintaining the structural and functional integrity of the intestine through interferon signals.
“In effect, the immune system was saying: ‘We may not be able to get rid of this worm, but we’re not going to let it destroy our house,’” says Susan Westfall, a postdoctoral fellow in King’s lab and lead author of the study.
The Forgotten Defenders: Stromal Cells in a New Light
Traditionally, immunology has focused on white blood cells: T cells, B cells, macrophages, and their ilk. But King’s team turned their attention to the often-overlooked stromal cells. These are the cells that form the architectural framework of the intestine—the scaffolding that holds everything together, facilitates tissue repair, and shapes how immune cells move and function within organs.
Stromal cells are not passive bystanders. In recent years, research has begun to show that they act as active participants in immune responses, especially during chronic disease. This study provides the clearest evidence yet that stromal cells are essential collaborators in immune defense—not by killing pathogens, but by buffering the damage they cause.
“Without interferon signaling, the stromal cells fail to adapt,” explains King. “They can’t repair tissue properly or maintain the intestinal barrier, and the whole system begins to collapse.”
This opens an entirely new dimension of host-pathogen interaction—one focused on tissue tolerance rather than eradication. It also raises fascinating possibilities for treating diseases where tissue damage, rather than infection itself, is the main driver of illness.
An Organ Under Siege: Why Gut Health Is Fragile
To understand the significance of this discovery, one must appreciate the daily onslaught faced by the intestine. It is arguably the most assaulted organ in the human body, exposed to a continuous stream of microbes, food particles, foreign chemicals, and even internal metabolic waste. Every bite we eat introduces not only nutrients but also a mix of potential irritants and immune triggers.
The 2019 Global Burden of Disease Study underscores the consequences: digestive disorders afflict more than a third of the global population. Enteric infections are the leading cause of death from digestive diseases worldwide, especially in children in low-resource settings.
Add to this the modern lifestyle: overuse of antibiotics, highly processed diets, air and water pollutants, and the rise in autoimmune and inflammatory conditions. The gut is taking the brunt of it all.
“The modern world is very harsh on the gut,” says King. “And we haven’t had the evolutionary time to adapt to all these changes. Our research helps explain one way the immune system has evolved to cope with long-term threats without tearing the organ apart.”
Rethinking Treatments: Tolerance, Not Just Elimination
Western medicine has largely been built on a war metaphor—find the pathogen and kill it. But this discovery challenges that framework.
“We tend to think of infection as something to eliminate at all costs,” says Westfall. “But in many parts of the world, people are living with parasites and pathogens that are not going away anytime soon. The question then becomes: How do we maintain health in spite of infection?”
By shifting focus from the immune system’s offensive weapons to its protective infrastructure, King’s team is helping inaugurate a new era in immunology—one that recognizes the importance of resilience and repair, not just eradication.
This shift could have profound implications. In inflammatory bowel diseases like Crohn’s and ulcerative colitis, for example, immune cells mistakenly attack the intestinal lining. If therapies could mimic or enhance the protective interferon-stromal pathway, they might help prevent the fibrosis and tissue degradation that characterize these chronic conditions.
Similarly, in cancer and fibrosis, uncontrolled stromal cell activation leads to pathological scarring and organ failure. Understanding how interferons moderate this process could lead to entirely new therapeutic strategies.
The Road Ahead: From Mice to Medicine
Of course, this research began in mice—and while the findings are powerful, translating them into human therapies will take time. Yet the fundamental insight is universal: the immune system is not simply a destroyer of invaders but also a guardian of tissue harmony.
King’s team is already collaborating with researchers studying human organoids—lab-grown miniature intestines—to explore how the interferon-stromal axis operates in human tissues. They are also investigating whether vaccines could be designed not just to prevent infection but to promote tissue tolerance, especially in regions where helminth infections remain endemic.
“If we can harness these protective mechanisms, we might be able to design therapies that protect people even when elimination isn’t possible,” says King. “That could be a game-changer for global health.”
Science Rooted in Curiosity—and Collaboration
What makes this study especially notable is not just the result but the process. The breakthrough was the product of interdisciplinary collaboration and open-ended curiosity.
“We started by asking a simple question: Why isn’t the intestine falling apart in the presence of worms?” says King. “The answers came not from one field, but from many—immunology, cell biology, parasitology, and even engineering.”
Contributors to the study included stem cell expert Alex Gregorieff, immunologist Maziar Divangahi, infectious disease specialist Donald Vinh, and researchers from around the globe. Their efforts illustrate the value of breaking down academic silos and following scientific intuition wherever it leads.
A New Lens on Health and Disease
This discovery may not make headlines like a new drug or vaccine, but its impact could be just as revolutionary. It invites us to reimagine what immune protection means, especially in a world where complete elimination of disease is not always possible. It reframes the gut not as a helpless victim of infection but as a dynamic ecosystem—one that can be protected from the inside out.
In doing so, it rekindles an ancient truth too often forgotten in modern medicine: health is not merely the absence of disease, but the presence of balance.
And sometimes, to defend ourselves best, we don’t need to fight harder—we need to heal smarter.
Reference: Susan Westfall et al, A type 1 immune-stromal cell network mediates disease tolerance against intestinal infection, Cell (2025). DOI: 10.1016/j.cell.2025.03.043