In the shadowy battlefields of neurodegenerative disease, where tangled proteins, lost memories, and dying neurons define the terrain, a new discovery has lit a beacon of hope. Scientists at UT Southwestern Medical Center have identified a protein that may act as a master control switch for one of the brain’s most enigmatic and double-edged processes—reactive gliosis.
The findings, published in the prestigious journal Neuron, point to a little-known gene called Gadd45g as a powerful upstream regulator of this complex response. Long viewed as a consequence of neurological damage, reactive gliosis is now emerging as a possible driver of it. The implications are staggering: if scientists can control this protein, they may be able to alter the trajectory of diseases like Alzheimer’s, Parkinson’s, Huntington’s, and beyond.
The Hidden Players of the Brain
For centuries, neurons—the electric messengers of the nervous system—have stolen the spotlight in neuroscience. But in the last few decades, glia have stepped from the shadows.
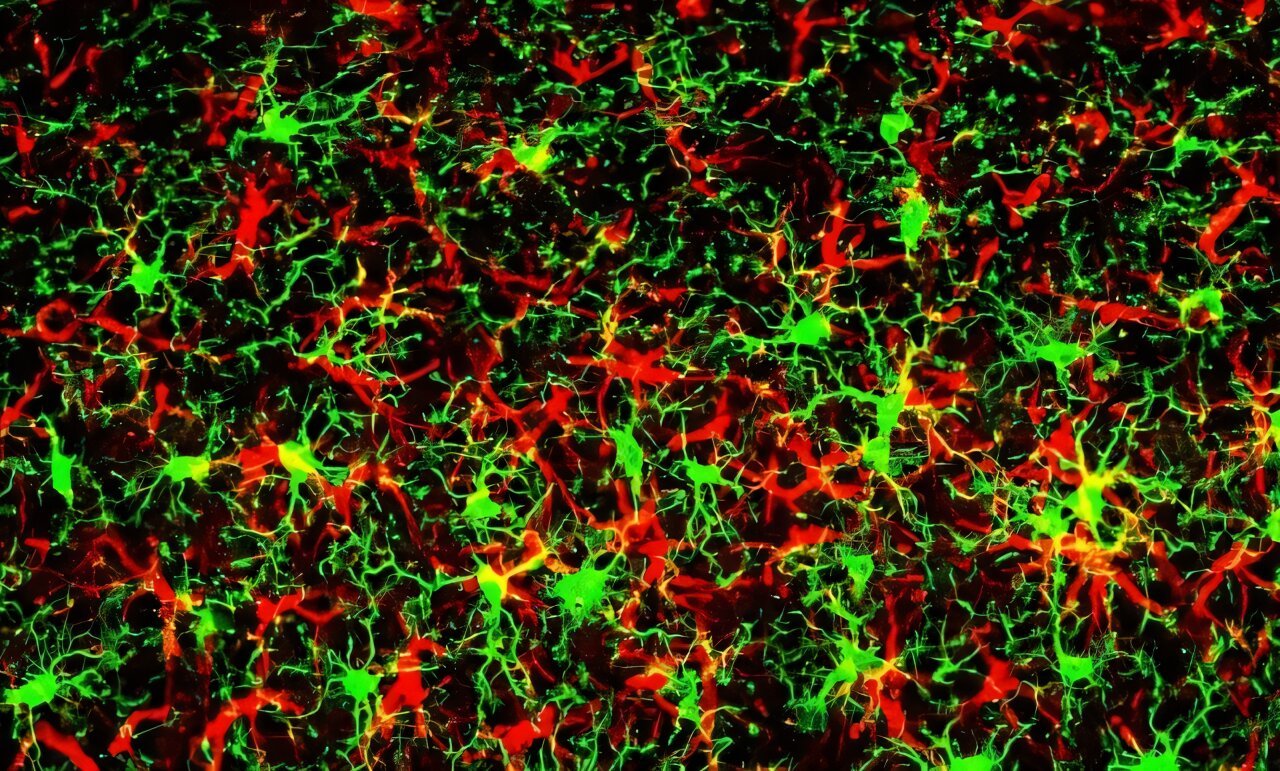
Glial cells, whose name comes from the Greek for “glue,” were once thought to be mere support staff for the brain’s star performers. Today, they are understood to be sophisticated, essential participants in nearly every aspect of brain function. Astrocytes, named for their star-like shape, regulate neurotransmitters, modulate blood flow, and maintain the blood-brain barrier. Microglia, meanwhile, act as the immune sentinels of the brain, scanning for infections, clearing debris, and pruning synapses.
Together, glia make up more than half the volume of the central nervous system. And when the brain is injured—whether by trauma, stroke, or disease—these cells launch a coordinated response known as reactive gliosis. It is both heroic and, potentially, tragic.
The Double-Edged Sword of Gliosis
Reactive gliosis is the glial cells’ equivalent of going into battle mode. The astrocytes swell, multiply, and change their gene expression patterns. They release a cocktail of proteins designed to protect the brain—sealing off damaged areas, scavenging toxic byproducts, and secreting neurotrophic factors that help neurons survive. This response can stabilize the central nervous system, allowing it to continue functioning under stress.
But there’s a dark side.
When reactive gliosis becomes prolonged or excessive, the very cells meant to protect the brain begin to hurt it. They release inflammatory cytokines that worsen neuronal injury. They form physical and biochemical barriers that prevent the regrowth of axons—the slender extensions that neurons use to connect with each other. They reduce the number of synapses, impairing communication between brain cells. And in severe cases, they can even trigger apoptosis, or programmed cell death, in neurons.
For years, scientists knew that reactive gliosis was a hallmark of neurodegenerative conditions—but it wasn’t clear whether it was a symptom or a cause. The new findings suggest it may be both, and that a single gene—Gadd45g—may lie at the heart of this Janus-faced phenomenon.
The Gene That Listens to Stress
The journey to Gadd45g began in an unexpected place: the immune response. Dr. Chun-Li Zhang, a Professor of Molecular Biology at UT Southwestern, and his team had long been interested in how the brain adapts to stress. Together with research scientist Dr. Tianjin Shen, they combed through gene activity databases of astrocytes exposed to inflammation, particularly after exposure to bacterial toxins that simulate infection.
One gene stood out. Gadd45g, short for “Growth arrest and DNA damage-inducible protein 45 gamma,” lit up like a flare in astrocytes under stress. It had previously been studied in cancer biology, where it was known to respond to DNA damage and cellular stress, but its role in the healthy brain remained a mystery.
To explore that role, Zhang’s team genetically engineered mice to overproduce GADD45G, the protein product of the Gadd45g gene, specifically in their astrocytes. The results were stunning—and a little alarming.
Gliosis on Command
When astrocytes in the mouse brain were programmed to overexpress GADD45G, they underwent full-blown reactive gliosis—even in the absence of disease or injury. More surprising still, the glial cells began secreting chemical messengers that induced gliosis in neighboring, unmodified cells. In other words, once activated, the GADD45G pathway could spread like wildfire, transforming the brain’s support network into a pro-inflammatory, neuron-damaging engine.
To confirm these findings outside of living animals, the researchers turned to a petri dish. When neurons were grown alongside astrocytes engineered to produce GADD45G, the neurons lost synapses and showed signs of inflammation. Reactive gliosis wasn’t just a reaction—it was a cause of neuronal dysfunction.
But was this process relevant to human disease?
The Alzheimer’s Connection
To answer that question, Zhang’s team turned to a well-established mouse model of Alzheimer’s disease, genetically engineered to develop amyloid-beta plaques, the sticky, pathological protein clusters that are a defining feature of the condition.
In these mice, Gadd45g expression was significantly elevated. The more amyloid plaques the mice accumulated, the more active the Gadd45g gene became. The researchers then modified some of the Alzheimer’s mice to overexpress GADD45G even further—with catastrophic results.
These mice not only developed twice as much amyloid-beta in their brains, but they also suffered from accelerated inflammation, worse memory loss, and greater cognitive decline. It was as though GADD45G was pressing its foot down on the accelerator of neurodegeneration.
In a final and perhaps most hopeful experiment, the researchers reversed the process. By selectively deleting Gadd45g in astrocytes, they were able to dramatically reduce amyloid-beta accumulation. Even more remarkable, the Alzheimer’s model mice performed better in memory and learning tests than their unmodified counterparts. Somehow, turning off this single gene had rescued cognition.
The Master Switch Hypothesis
The evidence pointed to a powerful conclusion: GADD45G is a master regulator of reactive gliosis, capable of flipping astrocytes into a disease-promoting state and initiating a cascade that leads to inflammation, synapse loss, and cognitive decline.
“This discovery gives us a target—a molecular switch we may be able to control,” said Dr. Zhang. “If we can find drugs or gene therapies that modulate GADD45G activity, we might be able to slow or even reverse the progression of neurodegenerative disease.”
It’s a bold vision, but one grounded in a decade of painstaking molecular biology. Scientists have long sought a way to tame gliosis without completely disabling the protective functions of glial cells. GADD45G, with its central role in stress sensing and gliosis activation, could offer a precision lever—one that nudges astrocytes back toward their protective roles and away from their destructive ones.
From Bench to Bedside
Of course, translating this discovery into treatments will take time. Gene therapy approaches are still in their infancy, particularly in the brain. And while the team’s results are compelling in mice, human brains are far more complex, and clinical trials would require careful design to avoid unintended effects.
Still, the road ahead is clearer now than it was. The discovery of GADD45G’s role opens up new research avenues—not only for Alzheimer’s but also for other conditions marked by gliosis, including Parkinson’s, Huntington’s, multiple sclerosis, traumatic brain injury, and even certain psychiatric disorders.
Already, pharmaceutical companies are showing interest in molecules that can modulate the GADD45G pathway. Zhang’s lab is collaborating with chemists and drug developers to screen for small-molecule inhibitors or RNA-based therapies that might one day be delivered directly to astrocytes.
Rethinking Neurodegeneration
The broader implication of this work is that neurodegeneration may not be solely a neuron problem. For decades, treatments have focused on saving neurons from death—by targeting amyloid, tau, or other neuronal markers. But this research suggests that glial cells, particularly astrocytes, may be the real gatekeepers of brain health.
If we want to stop Alzheimer’s before it starts, we may need to intervene not in the neurons, but in the neighborhood around them—the glial ecosystem that nurtures or neglects them, depending on its molecular instructions.
And those instructions, it seems, are written at least in part by a small gene with a big job: Gadd45g, the stress sensor that may be the brain’s most dangerous—or most valuable—switch.
Reference: Tianjin Shen et al, GADD45G operates as a pathological sensor orchestrating reactive gliosis and neurodegeneration, Neuron (2025). DOI: 10.1016/j.neuron.2025.04.033