In the shadowy crypts of the intestine, deep beneath the surface of the gut lining, a quiet drama unfolds every day—one that determines our ability to absorb nutrients, defend against pathogens, and recover from damage. Stem cells residing in these crypts must constantly decide what kind of cell to become. Should they become absorptive cells to soak in vital nutrients? Or secretory cells that produce the mucus, peptides, and hormones that fortify the gut’s frontline defenses?
Now, scientists at Memorial Sloan Kettering Cancer Center (MSKCC) have discovered that this pivotal decision is governed by more than just genes and proteins—it’s shaped by the cell’s metabolism itself. In a landmark study published in Nature, researchers have identified a metabolic switch—an enzyme known as OGDH—that helps determine whether intestinal stem cells grow into absorptive or secretory cells. Tweak this switch, they found, and you can guide stem cells toward healing in times of disease.
The implications ripple outward from the lab bench to the clinic: a new way to think about regeneration, inflammation, and possibly even treatment for diseases like Crohn’s and ulcerative colitis.
The Intestine’s Balancing Act
The gut epithelium—the single-cell layer lining the intestines—is one of the fastest regenerating tissues in the human body. Every few days, its entire surface is renewed, thanks to a cadre of intestinal stem cells located in crypts at the base of the epithelial folds.
These cells divide and differentiate into two major categories: absorptive enterocytes, which maximize nutrient uptake, and secretory cells, including goblet cells (which secrete protective mucus), Paneth cells (which release antimicrobial peptides), and enteroendocrine cells (which modulate gut function through hormones). Maintaining the right balance between these cell types is critical for gut health.
“Injury and inflammation can tip this balance,” explains Dr. Lydia Finlay, one of the study’s senior authors. “In diseases like colitis, secretory cells often become depleted, compromising the gut’s ability to defend itself and heal.”
While scientists have long known that transcription factors and signaling pathways like WNT, Notch, and BMP orchestrate this process, what remained unclear was how metabolic cues—internal biochemical signals that reflect a cell’s energy state—might influence stem cell fate.
A Closer Look at Cell Metabolism
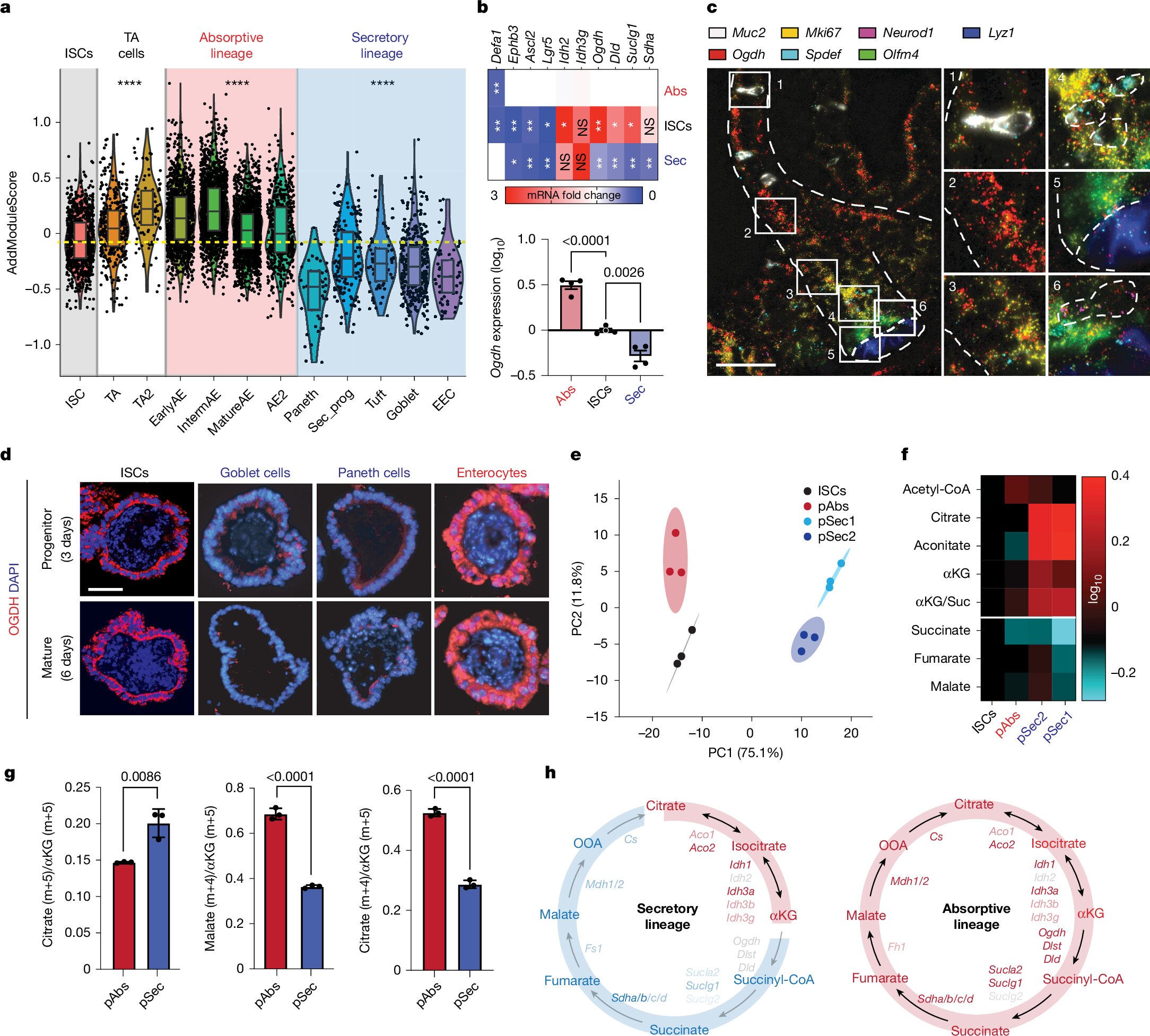
To dig into this question, the research team engineered sophisticated mouse models and intestinal organoids—miniaturized, lab-grown versions of the gut—to study stem cell behavior in a controlled environment. They then performed metabolomic profiling, which allowed them to catalogue hundreds of metabolites inside different types of stem cell progeny.
What they found was striking: progenitors destined to become absorptive cells were flush with ATP and other biosynthetic ingredients, signaling a high-energy state primed for cell division and expansion. Meanwhile, those leaning toward the secretory lineage had elevated levels of citrate, aconitate, and α-ketoglutarate (αKG)—intermediates in the tricarboxylic acid (TCA) cycle, also known as the Krebs cycle.
But these secretory progenitors curiously lacked downstream TCA-cycle intermediates like fumarate and malate, suggesting a metabolic bottleneck. The missing link? The enzyme OGDH, which catalyzes the conversion of αKG to succinyl-CoA—a key step in energy production.
OGDH: The Metabolic Gatekeeper
When researchers suppressed OGDH in stem cells, something remarkable happened. The cells began to accumulate αKG, and rather than proliferating as absorptive cells, they shifted toward the secretory lineage. Importantly, this did not trigger cell death—the cells simply chose a different fate.
Conversely, when OGDH was suppressed in absorptive progenitors, those cells began to die. Without OGDH, their mitochondria ran dry—ATP dropped, fumarate and malate were depleted, and the cells could no longer meet their biosynthetic demands.
To confirm the centrality of αKG, the team supplemented cells with a cell-permeable αKG analog, which mimicked the effects of OGDH inhibition. Again, the secretory fate was favored. This confirmed αKG’s role not just as a byproduct of metabolism, but as a driver of cell identity.
Even more intriguingly, the researchers discovered that secretory progenitors with high αKG levels showed elevated DNA hydroxymethylation, particularly at genes involved in secretory differentiation. This epigenetic signature, marked by the chemical 5-hydroxymethylcytosine (5hmC), suggested that αKG was rewiring the cell’s identity at the level of DNA structure and gene expression.
Healing the Inflamed Gut
To see whether this mechanism played a role in real-world disease, the scientists turned to a mouse model of colitis, a condition that mimics human ulcerative colitis. During inflammation, they observed that OGDH levels increased and αKG dropped—a metabolic shift that favored absorptive cells but impaired the gut’s secretory defense.
But when they suppressed OGDH or administered αKG supplements, the balance was restored. The number of goblet and Paneth cells increased, epithelial integrity improved, and markers of inflammation declined. Even levels of 5hmC rose in intestinal tissue, confirming epigenetic reprogramming.
“These findings are a compelling proof-of-concept,” said Dr. Alexandra Llorens, co-author of the study. “By tuning metabolism, we can steer regeneration in the gut—and possibly accelerate healing in patients with inflammatory bowel disease.”
A New Frontier in Regenerative Medicine
The broader implications of this work extend beyond the gut. In recent years, scientists have begun to appreciate the deep connection between metabolism and cell fate. But until now, most evidence came from early embryonic cells or in vitro studies. This research brings metabolic control to the forefront of tissue regeneration in living organisms.
It also raises tantalizing clinical possibilities. Could targeted OGDH inhibitors be used to promote secretory cell regeneration in patients with colitis? Could αKG supplementation enhance recovery from intestinal injuries or chemotherapy?
The answers remain speculative for now. Human trials are a long way off, and safety concerns will need to be carefully addressed. Still, the study opens new doors in the quest to heal the gut from within.
“We often think of metabolism as passive—a reflection of what the cell is doing,” says Dr. Finlay. “But what we’re seeing here is the opposite. Metabolism is actively shaping what the cell becomes.”
Beyond the Gut
The idea that a single metabolic enzyme could tip the scales of stem cell differentiation challenges conventional wisdom—and suggests similar mechanisms may exist in other organs. Could tweaking metabolism help regenerate lung tissue, liver cells, or even neurons?
Already, research in fields like cancer metabolism, immunometabolism, and neuroregeneration hints at this potential. As scientists continue to map the biochemical pathways of stem cell fate, the line between metabolism and identity grows ever blurrier.
The gut, with its rapid turnover and regenerative prowess, has once again proven to be a window into the body’s most profound mysteries. And thanks to the work of researchers at Memorial Sloan Kettering, that window is now a little clearer—and a lot more hopeful.
References: Almudena Chaves-Perez et al, Metabolic adaptations direct cell fate during tissue regeneration, Nature (2025). DOI: 10.1038/s41586-025-09097-6
Ram P. Chakrabarty et al, Mitochondrial molecule has unexpected role in tissue healing, Nature (2025). DOI: 10.1038/d41586-025-01583-1