In the quiet hum of a Harvard lab, scientists have done something extraordinary. With a handful of genetic instructions and a flash of synthetic biology, they’ve coaxed stem cells to become human microglia—the brain’s immune sentinels—in just four days. This groundbreaking feat, led by a team at the Wyss Institute at Harvard University and Harvard Medical School, could transform how we study—and someday treat—neurodegenerative diseases like Alzheimer’s, Parkinson’s, and ALS.
The implications ripple far beyond the petri dish. Because when microglia fail, the brain can spiral into chaos. And until now, growing these elusive cells in the lab has been an exercise in frustration—slow, costly, and only partially effective.
But this new approach, blending developmental biology, AI-driven analytics, and a powerful gene-regulating platform called TFome™, may finally crack the code.
The Brain’s Janitors, Architects, and Bodyguards
Microglia may not be as famous as neurons, but they are indispensable. These tiny, vigilant immune cells make up about 10% of all cells in the brain and spinal cord. They are its janitors, sweeping away dead cells and infectious debris; its architects, helping sculpt neural circuits during development; and its bodyguards, protecting against invaders.
But when they go rogue or lose functionality, the results can be catastrophic. Damaged microglia have been linked to nearly every major neurodegenerative disease. Instead of cleaning up dangerous protein clumps like amyloid plaques in Alzheimer’s or TDP-43 in ALS, dysfunctional microglia often inflame the brain and speed up degeneration. Sometimes, they even turn against healthy neurons.
Crucially, inflammation driven by faulty microglia may start before any visible damage appears—making these cells a tantalizing early warning system and potential therapeutic target.
The Supply Problem: Why Growing Microglia Has Been So Hard
Despite their importance, scientists have struggled to get their hands on microglia. Extracting them from human brains requires invasive biopsies. Rodent microglia—long used as a stand-in—are simply not the same. And attempts to grow human microglia from induced pluripotent stem cells (iPSCs) have been agonizingly slow and inefficient, taking over a month and costing a fortune.
“Time is everything when you’re studying a dynamic, inflammatory disease like Alzheimer’s or ALS,” said Dr. Jenny Tam, co-lead of the study and Director of the Synthetic Biology platform at the Wyss Institute. “Waiting 35 days to get cells that are only somewhat functional has held back the field for years.”
But what if we could press fast-forward?
The Breakthrough: Six Genes, Four Days, One Powerful Idea
In a new study published in Nature Communications, researchers led by synthetic biology pioneer Dr. George Church have dramatically shortened the process. Their secret weapon: TFome™, a synthetic biology platform that uses transcription factors (TFs)—the master switches of gene expression—to program cells.
Think of TFome™ like a genetic orchestra. Instead of waiting for cells to naturally “decide” to become microglia over weeks, the researchers handed them a pre-written score and told them exactly what to play—and when.
The team started with a smartly curated set of 40 transcription factors suspected of being key to microglia identity. Then, using an iterative trial-and-error process and advanced single-cell RNA sequencing, they identified which combinations best triggered microglia-like gene expression.
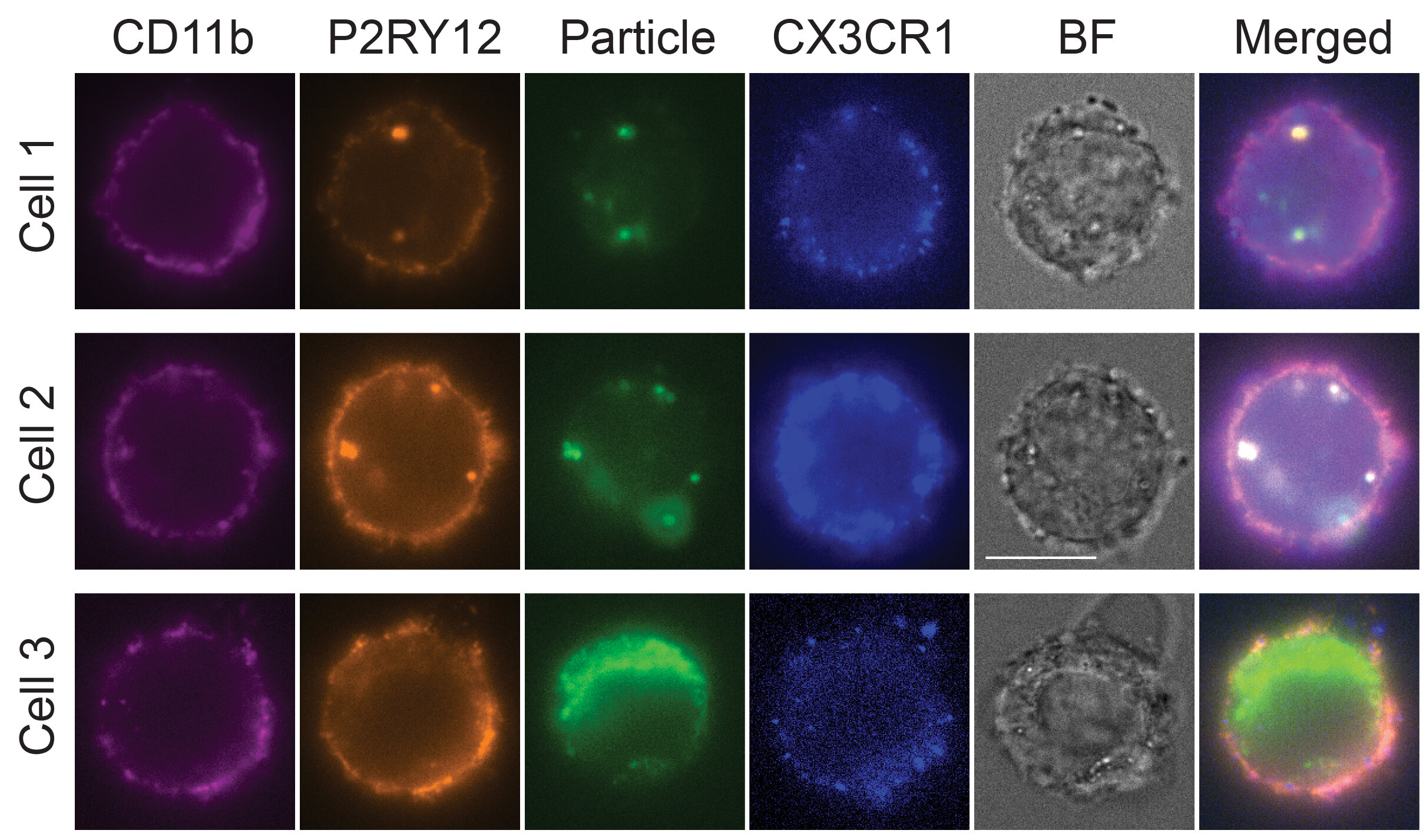
After several rounds of optimization, they landed on a cocktail of six transcription factors—SPI1, CEBPA, FLI1, MEF2C, CEBPB, and IRF8. These were enough to convert stem cells into microglia-like cells with astonishing speed and fidelity. Within four days, the cells not only looked like microglia—they acted like them.
Functional, Not Just Facsimiles
To test how well these lab-grown microglia mimicked their real-world counterparts, the team put them under stress. Exposing the cells to interferon gamma—a molecule that spikes during brain infections—triggered a robust immune response.
More compellingly, when challenged with TDP-43, the same protein that clumps in ALS patients’ neurons, the cells activated gene expression patterns matching human microglia’s behavior during disease.
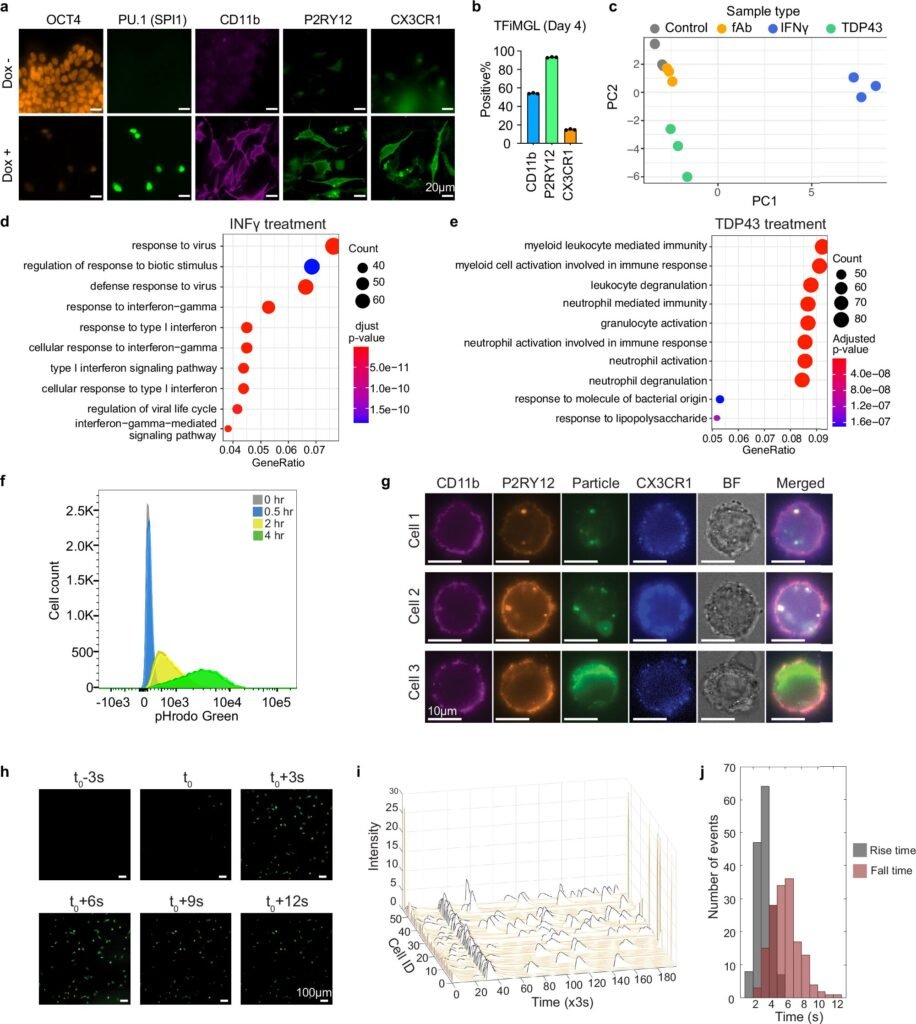
“This wasn’t just mimicry. These cells were doing the work of microglia,” said Dr. Songlei Liu, the study’s first author and now a scientist at nChroma Bio. “They were sensing threats and responding like soldiers on patrol.”
From Organelles to Organoids: A Bigger Vision
This leap in cell manufacturing wasn’t born in isolation. It traces back to earlier work by Church’s team on brain organoids—miniature, lab-grown models of the human brain. These “brains in a dish” had neurons, support cells, even vasculature—but they were missing something critical: microglia.
“You can’t study neuroinflammation in a brain model that lacks immune cells,” explained Dr. Katharina Meyer, one of the study’s co-authors. “And you can’t understand diseases like ALS or Alzheimer’s without looking at that interaction.”
With the new ability to generate functional microglia quickly and reliably, those organoids can now host all the major cellular players found in the human brain—ushering in a new era of disease modeling and drug discovery.
From Petri Dish to Patient?
It’s still early days. The six-TF cocktail is a huge step forward, but Church’s group believes they can make it even better—by fine-tuning the timing and dosage of each TF, or by identifying subtypes of microglia specialized for different brain regions or disease states.
What’s more, this breakthrough is part of a broader ambition. In 2021, Church’s team and co-authors Alex Ng and Parastoo Khoshakhlagh created a massive library of over 1,700 human transcription factors. Their startup, GC Therapeutics, aims to turn these recipes into next-generation cell therapies for everything from diabetes to mental health conditions.
Imagine off-the-shelf immune cells tailored to treat ALS. Or brain organoids that reveal the earliest molecular whispers of Parkinson’s. Or a personalized drug screening platform powered by your own microglia, derived from your own iPSCs.
This is no longer science fiction. The TFome™ platform, and the microglia breakthrough it just enabled, bring that vision closer.
Why It Matters
As our population ages and neurodegenerative diseases surge, the need for better models, earlier diagnostics, and smarter therapies has never been more urgent. Microglia are no longer passive bystanders in these diseases—they are active players, both villains and potential saviors.
This new research from the Wyss Institute doesn’t just give us faster microglia. It gives us functional, human-like microglia, on demand. It peels back the curtain on the complex dance between genes, cells, and disease—and gives us the tools to join in.
It also reflects something deeper: a shift in how we approach biology. Rather than waiting for nature to reveal its secrets, scientists are now writing new instructions, building custom cell types, and guiding development with the precision of engineering and the insight of medicine.
Or as George Church puts it, “We’re not just observing life. We’re programming it.”
And that, more than anything, might be the start of a revolution.
Reference: Songlei Liu et al, Iterative transcription factor screening enables rapid generation of microglia-like cells from human iPSC, Nature Communications (2025). DOI: 10.1038/s41467-025-59596-3