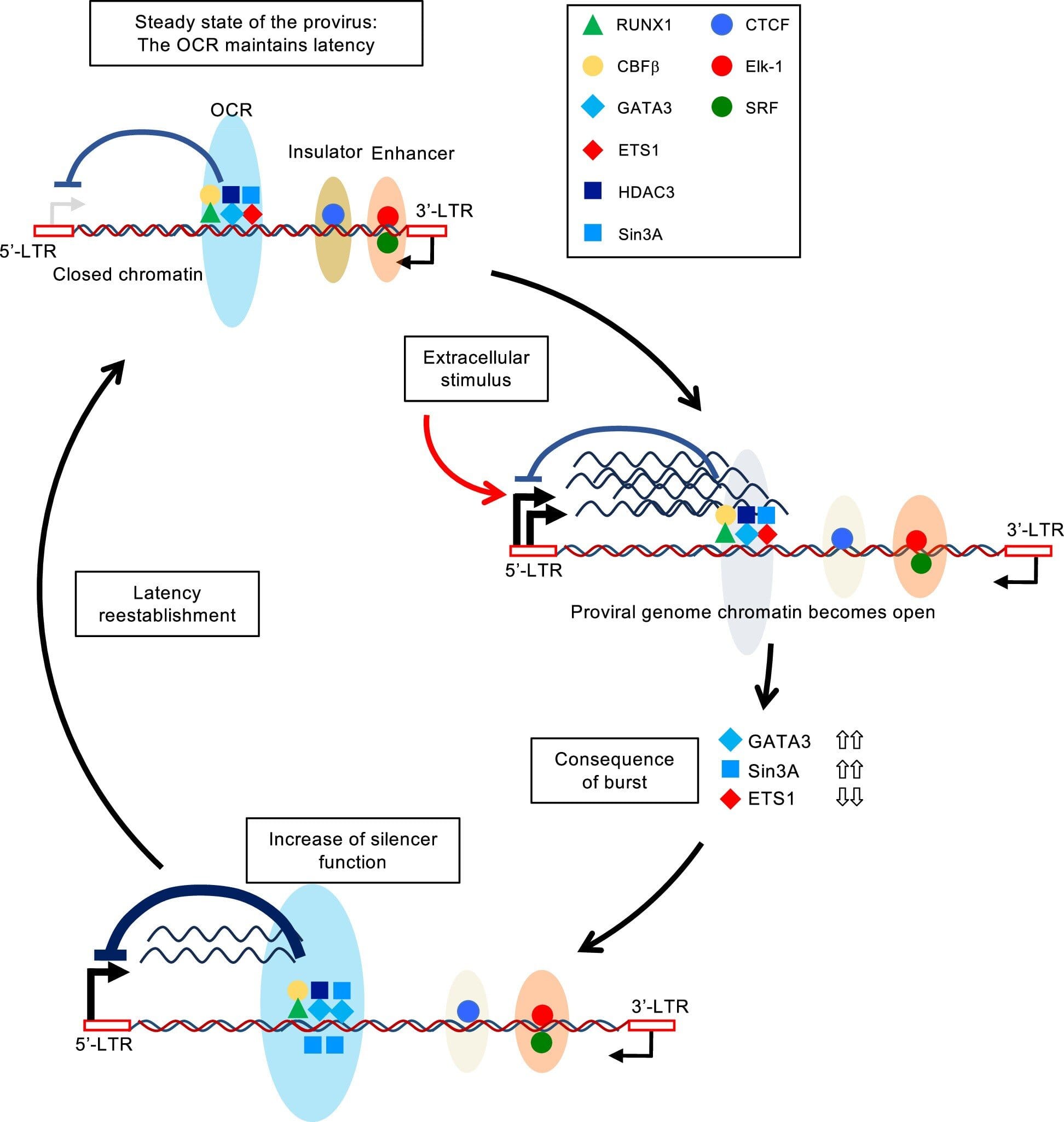
In a breakthrough study that may reshape our approach to treating viral cancers and HIV, scientists at Kumamoto University have discovered a genetic “silencer” element that enables the human T-cell leukemia virus type 1 (HTLV-1) to remain hidden and dormant inside the body. Their findings, published in Nature Microbiology, not only deepen our understanding of how HTLV-1 silently persists for decades but also open the door to potential new therapies for other retroviruses—including HIV.
HTLV-1 is no ordinary virus. While most people infected with it never show symptoms, it carries the potential to cause adult T-cell leukemia/lymphoma (ATL), an aggressive cancer with devastating outcomes. For years, scientists have puzzled over how the virus can evade the immune system for so long, lying in wait within the body’s cells without alerting the defenses. The answer, it turns out, lies in a clever piece of viral self-regulation.
Cracking the Code of Viral Silence
The research team, led by Professor Yorifumi Satou of the Joint Research Center for Human Retrovirus at Kumamoto University, homed in on a tiny region within the HTLV-1 genome—a stretch of genetic material that acts like a mute button for the virus. This “silencer” element, previously unknown, appears to be critical for the virus’s stealth mode.
What the scientists found is that the silencer doesn’t work alone. It recruits a protein complex from the host—specifically, RUNX1, a transcription factor known for its role in controlling gene activity. When RUNX1 binds to this silencer region, it essentially puts the brakes on HTLV-1, stopping it from making proteins that would otherwise expose it to the immune system.
In essence, the virus has evolved a built-in mechanism to regulate its own invisibility. As long as the silencer remains intact and active, the virus stays under the radar.
The Cost of Silence—and the Risk of Awakening
But what happens if the silencer is damaged or removed? The researchers explored this by deleting or mutating the silencer region in laboratory models. The results were striking: without the silencer, the virus reawakened, becoming more active, expressing its genes more freely—and, importantly, triggering a stronger immune response.
This suggests that while the silencer helps the virus survive quietly, it’s also its Achilles’ heel. If scientists can learn how to disrupt or bypass this silencing mechanism, they might be able to flush out the virus from hiding, making it easier to target with therapies or vaccines.
For the millions of people silently carrying HTLV-1—especially in endemic regions like southwestern Japan, parts of Africa, the Caribbean, and South America—this discovery offers a glimmer of hope. Currently, there is no cure for HTLV-1 infection, and treatments for ATL remain limited and often ineffective. Understanding how the virus evades detection is a crucial step toward changing that.
Borrowing the Silencer to Tame HIV
The implications of this discovery don’t stop with HTLV-1. In a stunning twist, the team inserted the same silencer region into HIV-1, the virus responsible for AIDS. The effect was remarkable: HIV became less aggressive, showing reduced replication and less cell destruction in experimental settings.
This suggests that the HTLV-1 silencer, or a synthetic version of it, could potentially be used to push HIV into a more dormant state—a strategy that might one day be part of long-term viral suppression or even a functional cure.
The idea that one virus’s evolutionary trick could help us tame another is a testament to the interconnectedness of life—and the ingenuity of scientific discovery.
A Clever Enemy—and a New Therapeutic Target
“This is the first time we’ve uncovered a built-in mechanism that allows a human leukemia virus to regulate its own invisibility,” said Professor Satou. “It’s a clever evolutionary tactic, and now that we understand it, we might be able to turn the tables in treatment.”
Understanding how viruses manage latency has been a long-standing challenge in virology. For retroviruses like HTLV-1 and HIV, latency is both a blessing and a curse. It allows the virus to persist long-term in the host, but it also makes it extremely difficult to eradicate.
Antiviral drugs typically target active replication, but if a virus is latent—essentially asleep—there’s nothing to hit. That’s why many scientists are now looking for ways to either “shock and kill” the virus (waking it up so the immune system or drugs can destroy it) or “block and lock” it in a deep, irreversible sleep. This new research offers clues for both strategies.
New Doors Open in the Fight Against Viral Cancers
The discovery could have wide-reaching implications not only for infectious disease medicine but also for oncology, especially in regions heavily burdened by HTLV-1-associated cancers. ATL, the most common cancer linked to HTLV-1, is notoriously hard to treat and carries a poor prognosis. If scientists can find a way to disrupt the virus’s silence without harming the host, it might be possible to prevent or delay the progression to leukemia.
Additionally, understanding how HTLV-1 manipulates host transcription factors like RUNX1 might lead to targeted therapies that interrupt this partnership—effectively forcing the virus into the spotlight where it can no longer hide.
The Bigger Picture: Lessons from Viral Evolution
What makes this discovery especially fascinating is its evolutionary insight. Viruses, like all organisms, are shaped by the pressures of survival. HTLV-1, in its long history with humans (dating back thousands of years), has developed a sophisticated means of coexisting with its host—remaining silent, causing little trouble, and avoiding elimination.
This level of viral self-restraint is unusual. Most viruses either replicate rapidly or die trying. But HTLV-1 shows us that sometimes, survival means staying quiet—and in that quiet, becoming nearly invisible.
Now, by listening to the silence, researchers have found its source—and perhaps, its weakness.
Where Do We Go from Here?
The path forward is exciting but complex. Translating these findings into treatments will require years of research, clinical testing, and a deeper understanding of the silencer’s interactions with the human genome. But the discovery gives scientists a new target, a fresh strategy, and a powerful narrative: one where the very silence that protects a virus can become the key to its defeat.
This research also underscores the importance of supporting basic science—the kind that asks deep questions about how life works, without always knowing where the answers will lead. In this case, a quiet piece of viral DNA might hold the secrets to defeating some of the most persistent and deadly viruses known to humankind.
In the intricate chess match between humans and viruses, it seems we’ve just learned a critical move. And with that, hope begins to stir—not with a bang, but with a whisper.
More information: Kenji Sugata et al, Intragenic viral silencer element regulates HTLV-1 latency via RUNX complex recruitment, Nature Microbiology (2025). DOI: 10.1038/s41564-025-02006-7