It’s a story that begins, improbably, in the dusty corners of early 20th-century medicine, with a bacterium originally bred to fight tuberculosis. In 1921, scientists Albert Calmette and Camille Guérin developed a weakened strain of Mycobacterium bovis, naming it Bacillus Calmette-Guérin—BCG for short. Intended as a vaccine to protect children from TB, BCG went on to become one of the world’s most widely administered vaccines.
But what no one could have predicted was BCG’s second life—its transformation from a childhood shield into a stealthy weapon against cancer. Approved by the U.S. Food and Drug Administration in 1990 as the first immunotherapy for any form of cancer, BCG has, for more than 30 years, been the frontline treatment for non-muscle invasive bladder cancer. And unlike many therapies that come and go with the tide of new discoveries, BCG has stood its ground, still routinely administered in hospitals today.
Now, new research from Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center (MSK) is illuminating just how BCG performs its immunological magic—not just in the bladder, but deep within the bone marrow itself. This revelation is redefining the narrative of cancer immunotherapy and opening up startling new possibilities for how we might fight cancer systemically, rather than solely at its site.
The Bladder Therapy That Defied Expectations
For decades, BCG’s use in bladder cancer has rested on an elegant assumption: inject a weakened bacterium directly into the bladder, and the patient’s immune system wakes up. BCG was believed to draw the attention of immune cells to the bladder lining, inflaming the tissue, recruiting T cells, and priming them to recognize and kill tumor cells.
While it worked—often very well—the exact biological mechanism remained partly mysterious. Was the immune system reacting primarily to the cancer? Or was it more interested in the bacterium itself? And how localized was this effect?
As physician-scientist Dr. Michael Glickman, acting director of the Marie-Josée Kravis Center for Cancer Immunobiology at MSK, put it: “This is an example of a therapy that was proven to be clinically effective before we fully understood all the underlying mechanisms.”
That phrase—”before we fully understood”—is perhaps the defining legacy of BCG. But the new study, published in Cancer Cell, flips that legacy on its head. It shows that BCG doesn’t just stir up an immune response within the bladder. Instead, it rewires the immune system from the inside out—starting in the bone marrow, where immune cells are born.
Immune Training at the Source: The Bone Marrow Effect
The innate immune system, often thought of as the body’s first responder, is like the blunt but dependable security guard at the entrance of a nightclub. It doesn’t need to recognize faces—it just needs to detect trouble. Unlike the adaptive immune system, which learns and remembers specific threats, innate immunity is faster and more generalized. Historically, it was seen as untrainable, like a dog that knows only how to bark.
But that view is changing.
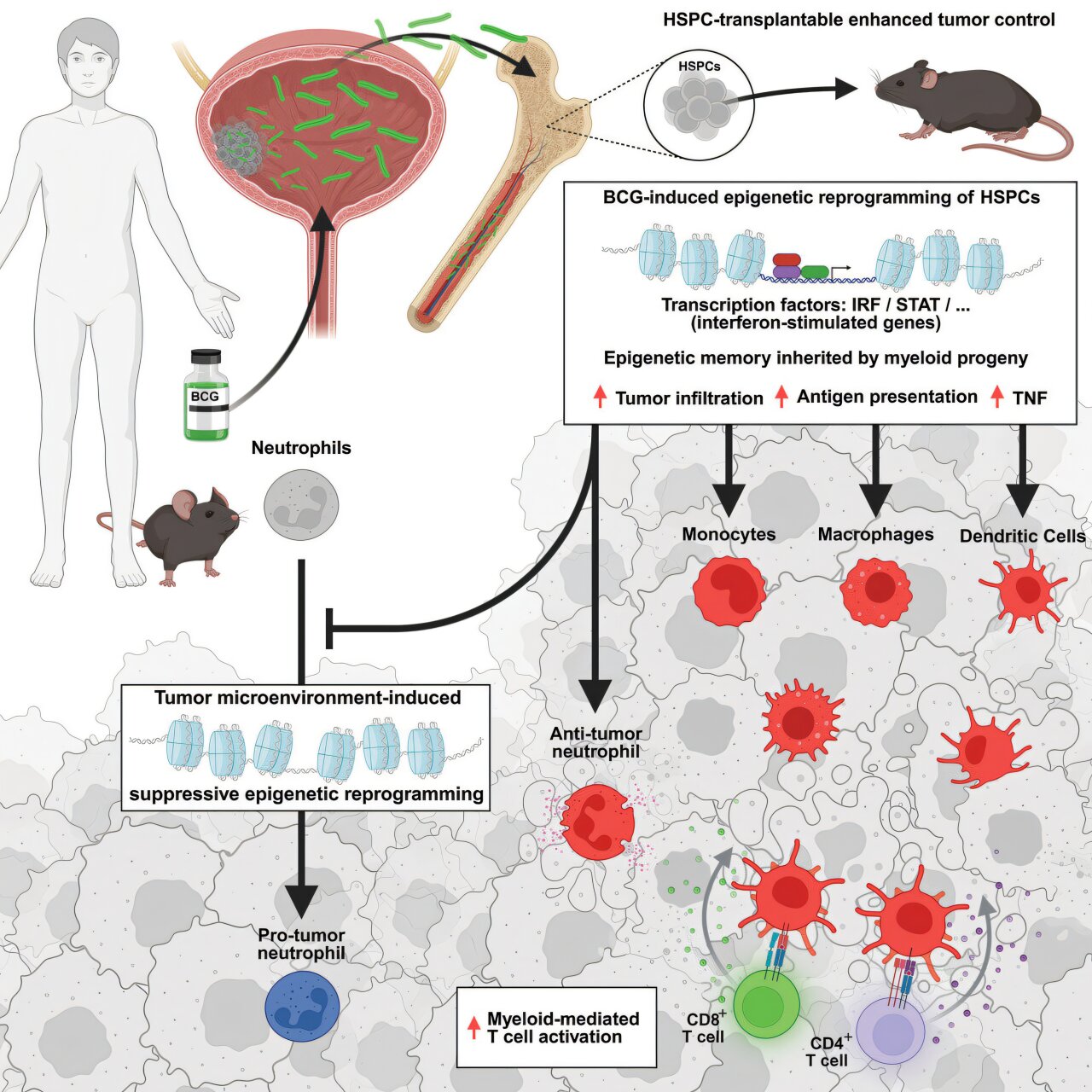
The new research shows that BCG has the surprising ability to “train” the innate immune system, making it more effective at detecting and destroying cancer cells. This training doesn’t happen in the bladder itself, but rather in the bone marrow, the birthplace of immune cells. When BCG is administered intravesically (directly into the bladder), it somehow migrates from the bladder to the bone marrow, where it reprograms hematopoietic stem and progenitor cells (HSPCs)—the ancestors of myeloid cells, which play a crucial role in immune surveillance and tumor destruction.
This was more than just a local skirmish. It was systemic immunological warfare.
To demonstrate this, researchers first turned to mice. After administering BCG to their bladders, the team was able to culture live BCG bacteria from their bone marrow—a stunning finding that revealed the bacteria’s journey far beyond its presumed perimeter.
But the real breakthrough came when they turned to humans.
Mining Blood for Bone Marrow Secrets
Studying bone marrow is typically a painful, invasive process. To understand how BCG affected human patients, the team needed a way to probe the immune system’s deepest layers—without drilling into people’s bones.
Enter PIE-seq (Progenitor Input Enrichment single-cell sequencing), a method developed by Dr. Steven Josefowicz’s lab at Weill Cornell Medicine. This technique allows scientists to analyze rare, primitive immune stem and progenitor cells from a simple blood draw, giving them insight into how the bone marrow is programming future generations of immune cells.
Using PIE-seq on bladder cancer patients who had received BCG therapy, the researchers observed significant genetic reprogramming in the stem cells responsible for generating myeloid immune cells. The message was clear: BCG was altering the fundamental architecture of the immune response.
Dr. Josefowicz, co-senior author of the study, explained the implications: “We now see that BCG therapy improves the innate immune system’s ability to fight cancer—not just in the bladder, but across the body. This helps explain why BCG has been so remarkably effective for so long, even without us fully understanding why.”
The Long Arm of Immune Memory
BCG’s systemic effects mirror another intriguing discovery: its ability to protect against unrelated infections. Previous studies have shown that BCG vaccination in elderly individuals, including nursing home residents, reduced the incidence of respiratory viral infections. The implication is that BCG somehow boosts the immune system’s general readiness—an immunological equivalent of a caffeine jolt.
What the new research suggests is that this “general alertness” isn’t limited to microbes. It might apply to cancer as well.
“We used to think the bladder was the battlefield, and BCG was the spark that started the fire there,” said Dr. Anthony Antonelli, co-first author of the study. “But what we’re seeing is that the fire is actually being lit deep in the marrow, and it’s igniting immune cells system-wide.”
This represents a fundamental rethinking of how localized cancer therapies might generate body-wide benefits—and how vaccines, or vaccine-like agents, might work not only preventatively but also therapeutically in cancer.
A Synergistic Partnership: BCG Meets Checkpoint Inhibitors
The study’s most exciting finding may lie in what happens when BCG is paired with another modern marvel of cancer treatment: checkpoint inhibitors. These therapies work by disabling molecular brakes that normally hold T cells in check, thereby unleashing them on tumors. But T cells don’t operate in a vacuum—they take their cues from the surrounding immune environment, particularly from myeloid cells.
And that’s where BCG comes in.
In mouse models of bladder cancer, combining BCG with checkpoint inhibitors led to superior outcomes: smaller tumors, longer survival, and a more robust immune response. The implication is that BCG-primed myeloid cells may enhance T-cell activation, effectively setting the stage for checkpoint inhibitors to do their job more effectively.
“It’s like giving the immune system a roadmap,” Dr. Josefowicz said. “BCG tells the myeloid cells what to look for and how to respond. Then the checkpoint inhibitors remove the obstacles preventing T cells from executing that response.”
A Broader Horizon: Could BCG Work Against Other Cancers?
One of the most tantalizing prospects raised by the new findings is whether BCG could be used to enhance immunotherapy across other forms of cancer—not just those that occur in the bladder.
Since BCG stimulates the bone marrow systemically, could intravesical administration or targeted delivery of BCG into other parts of the body act as a primer for cancers elsewhere? Could cancers of the lung, colon, or even brain be made more vulnerable to existing treatments by giving the immune system a kind of “BCG wake-up call”?
That remains speculative for now, but the research has already cracked open a door that many oncologists are eager to walk through.
“It’s early,” said Dr. Glickman. “But the idea that we could train the immune system at its source to enhance not just one therapy, but potentially many—that’s a compelling frontier.”
A Fusion of Old and New
There’s something poetic about a century-old vaccine being at the cutting edge of cancer treatment. In an era dominated by precision medicine, genetic profiling, and AI-driven diagnostics, BCG is a reminder that some of the most powerful tools in medicine are already in our hands—we just have to learn how to use them better.
And with new analytical techniques like PIE-seq, we’re doing just that. What was once a blunt instrument—inject bacteria into the bladder and hope—has become a precision tool for systemic immunological reprogramming.
BCG has transitioned from being a “miracle” born of accidental insight to a rationally understood therapy with mechanisms that are increasingly clear, testable, and improvable.
The Road Ahead
As always in science, each answer begets new questions. How exactly does BCG migrate from the bladder to the bone marrow? Can similar “training” effects be replicated using synthetic or non-bacterial agents? How long do these effects last? And can they be targeted to specific types of immune cells?
The answers may not just reshape bladder cancer treatment. They could redefine the blueprint for all immunotherapies.
Already, the research team is planning follow-up studies to test whether modifying the BCG strain—or engineering entirely new bacterial agents—could further enhance immune training. Others are exploring whether systemic delivery of BCG in low doses might boost immunity without risking adverse effects.
But even as we look ahead, it’s worth pausing to appreciate just how far we’ve come. From a TB vaccine to a cancer therapy, from a localized treatment to a systemic immune modulator—BCG’s story is one of medical reinvention at its finest.
Conclusion: Training Immunity, Treating Cancer
The story of BCG is a testament to science’s capacity for rediscovery. What began as a humble vaccine against tuberculosis has, over the course of a century, evolved into a cornerstone of cancer immunotherapy—one whose full potential is only now being revealed.
By showing that BCG reshapes immune function not just locally but at the root—the bone marrow—researchers have opened new doors to understanding and enhancing how our bodies fight cancer. It’s no longer just about what we kill, but what we teach the immune system to recognize, resist, and remember.
In a field where progress often hinges on billion-dollar biotech and ultra-precise gene editing, the enduring power of a 100-year-old bacterium is nothing short of inspiring.
Sometimes, the future of medicine is hidden in its past.
Reference: Andrew W. Daman et al, Microbial cancer immunotherapy reprograms hematopoiesis to enhance myeloid-driven anti-tumor immunity, Cancer Cell (2025). DOI: 10.1016/j.ccell.2025.05.002