Chronic illnesses, particularly those affecting the digestive system, have a profound impact on patients’ overall health, often leading to complications that extend far beyond the primary disease itself. One such example is Crohn’s disease, an inflammatory bowel disease (IBD) that primarily targets the intestines but has a range of systemic effects. Among these is iron-deficiency anemia, a prevalent and debilitating condition that is frequently observed in IBD patients, especially those with Crohn’s disease.
A recent study led by a team of biomedical scientists at the University of California, Riverside School of Medicine, sheds new light on the genetic underpinnings of this complication. The research, which focused on a genetic mutation associated with Crohn’s disease, has discovered a critical link between this mutation and the exacerbation of iron deficiency. This breakthrough could open new doors to better treatments for a common but often poorly addressed issue in IBD management.
Understanding the Connection Between IBD and Iron Deficiency
Inflammatory Bowel Disease is an umbrella term that includes two chronic conditions: Crohn’s disease and ulcerative colitis. These conditions cause chronic inflammation in the gastrointestinal tract, leading to symptoms such as abdominal pain, diarrhea, weight loss, and fatigue. While these are the most obvious symptoms, IBD can also trigger a range of other complications that affect various systems in the body. One of the most common and debilitating is anemia, a condition where the body lacks sufficient healthy red blood cells to carry adequate oxygen to tissues.
Iron-deficiency anemia is particularly widespread among those with IBD. The inflammation in the intestines can impair the absorption of nutrients, including iron, which is crucial for the production of hemoglobin, the protein in red blood cells responsible for oxygen transport. Even with oral iron supplements, many IBD patients continue to struggle with low iron levels, which contributes to chronic fatigue and diminished quality of life.
The recent study conducted by researchers at UC Riverside provides a compelling explanation for this phenomenon. Through analyzing serum samples from IBD patients, the scientists identified a specific genetic mutation that appears to exacerbate iron deficiency in these patients. The discovery offers an important new perspective on why some IBD patients fail to respond to traditional oral iron supplementation, opening the door for more effective treatment strategies.
The Role of the PTPN2 Gene in Iron Regulation
At the heart of this groundbreaking research is the PTPN2 gene, which codes for a protein known as protein tyrosine phosphatase non-receptor type 2 (PTPN2). This protein plays a critical role in regulating various cellular processes, including the immune response and the maintenance of intestinal integrity. In IBD patients, especially those with a loss-of-function mutation in the PTPN2 gene, researchers observed a disruption in the proteins responsible for regulating iron levels in the body.
Declan McCole, a professor of biomedical sciences at UCR and the lead investigator on the study, explained the significance of this finding. “This discovery sheds light on a critical mechanism that links a patient’s genetics to their ability to absorb and regulate iron, which is essential for maintaining healthy blood and energy levels,” McCole said. “Our findings offer an explanation for why some IBD patients remain iron-deficient despite oral supplementation.”
The study revealed that patients carrying the PTPN2 mutation exhibited significant disruptions in blood proteins involved in iron metabolism. This, in turn, caused problems with iron absorption in the intestines, even though the patients were taking iron supplements. This is a key discovery because it explains why some patients with IBD continue to experience chronic iron deficiency despite adhering to conventional treatments.
Animal Models: Genetic Mutation and Iron Absorption
To better understand the mechanisms at play, the researchers turned to animal models for further investigation. They specifically studied mice in which the PTPN2 gene was deleted. These genetically modified mice developed anemia and demonstrated an impaired ability to absorb iron from their food. Further analysis showed that the reduced iron absorption was due to decreased levels of a key protein responsible for iron uptake in the intestinal epithelial cells, which line the gut and facilitate nutrient absorption.
First author Hillmin Lei, a doctoral student in McCole’s lab, elaborated on the significance of these findings. “The only way the body can obtain iron is through intestinal absorption from food, making this discovery particularly significant,” Lei explained. “Disruption of this pathway by genetic variants like those in PTPN2 could help explain why some IBD patients fail to respond to oral iron therapy, a commonly prescribed treatment for anemia.”
This finding is particularly critical because it shows that even if a patient is consuming enough iron through their diet or supplements, their body may still be unable to properly absorb it due to genetic factors that affect intestinal function. In the case of PTPN2 mutations, the reduced expression of iron-absorbing proteins in the intestines leads to insufficient iron levels, exacerbating the anemia and contributing to the chronic fatigue commonly reported by IBD patients.
Implications for Treatment: Moving Beyond Oral Iron
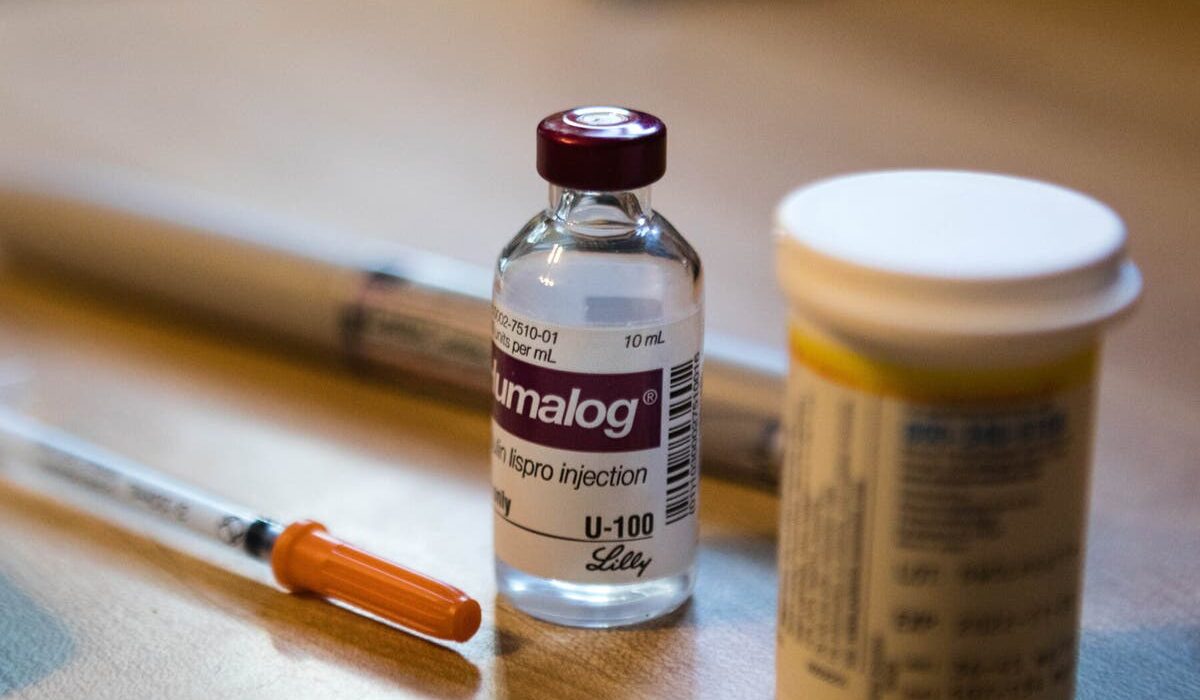
One of the most immediate and impactful consequences of this study is its potential to change the way iron deficiency and anemia are treated in IBD patients. As McCole pointed out, this discovery could pave the way for more targeted therapies that go beyond merely controlling inflammation in the gut. Specifically, patients who carry the loss-of-function mutation in PTPN2 may need to be treated with systemic intravenous (IV) iron supplementation instead of oral iron tablets.
“By identifying genetic factors like the PTPN2 mutation, we can begin to personalize treatment plans for IBD patients,” McCole said. “For those patients who have difficulty absorbing iron through their intestines, intravenous iron supplementation could be a much more effective treatment than oral supplements, which may be poorly absorbed due to the genetic disruption in their iron absorption pathway.”
This personalized approach to anemia treatment would not only improve the management of iron deficiency in IBD patients but could also help alleviate the chronic fatigue and other systemic symptoms that arise from low iron levels. For IBD patients, this could mean a better quality of life and more effective management of their disease.
Collaborative Efforts and International Reach
This research was not conducted in isolation but rather in collaboration with multiple institutions, further highlighting the significance and global impact of the findings. Researchers from the City of Hope, University Hospital Zurich, and the Swiss IBD Cohort contributed to the study, working together to analyze serum samples from IBD patients and conduct animal experiments.
The study, titled “PTPN2 Regulates Iron Handling Protein Expression in Inflammatory Bowel Disease Patients and Prevents Iron Deficiency in Mice,” was published in the International Journal of Molecular Sciences and has already sparked interest in the scientific community. By connecting genetic factors to iron absorption issues in IBD patients, the researchers have opened new avenues for investigation into the broader implications of genetic mutations on nutrient metabolism.
The international collaboration highlights the importance of cross-border research efforts in understanding complex diseases like IBD. By pooling resources and expertise from around the world, the research team was able to produce findings that could have a profound impact on how IBD and related complications are treated in the future.
The Road Ahead: Toward Precision Medicine
The findings from this study represent an exciting step forward in the understanding of how genetic mutations can influence the course of chronic diseases like Crohn’s disease. However, much more research is needed to fully understand the scope of genetic factors involved in IBD and their impact on various complications, such as anemia. This study lays the groundwork for future investigations into how other genetic variants might affect nutrient absorption or contribute to other IBD-related complications.
In the broader context of medicine, this research underscores the growing importance of precision medicine, where treatment is tailored to the individual based on their unique genetic makeup. By understanding how specific genetic mutations influence disease progression and treatment response, healthcare providers can offer more personalized and effective interventions.
As the field of genetic research continues to evolve, studies like this one demonstrate the potential for innovative therapies that address not only the symptoms of IBD but also the underlying genetic factors that contribute to these complications. The hope is that with further research, IBD patients will experience more effective treatments that help them maintain better health, reduce complications, and improve their overall quality of life.
Conclusion: A New Era for IBD Treatment
This groundbreaking study offers a crucial insight into the genetic factors that contribute to iron deficiency and anemia in IBD patients. By identifying the role of the PTPN2 gene in disrupting iron absorption, the researchers have provided an important piece of the puzzle in understanding how Crohn’s disease and other forms of IBD can cause systemic complications.
As we move forward, the research has the potential to transform the way iron-deficiency anemia is treated in IBD patients, offering more personalized and effective therapies. It is a reminder that the path to better treatment for chronic diseases often requires us to look beyond the obvious symptoms and into the genetic and molecular mechanisms that govern the body’s complex functions.
With continued research and collaboration, the hope is that patients with IBD will not only see improvements in their gut health but also in their overall well-being, thanks to advances in personalized medicine and targeted therapies.
Reference: Hillmin Lei et al, PTPN2 Regulates Iron Handling Protein Expression in Inflammatory Bowel Disease Patients and Prevents Iron Deficiency in Mice, International Journal of Molecular Sciences (2025). DOI: 10.3390/ijms26073356