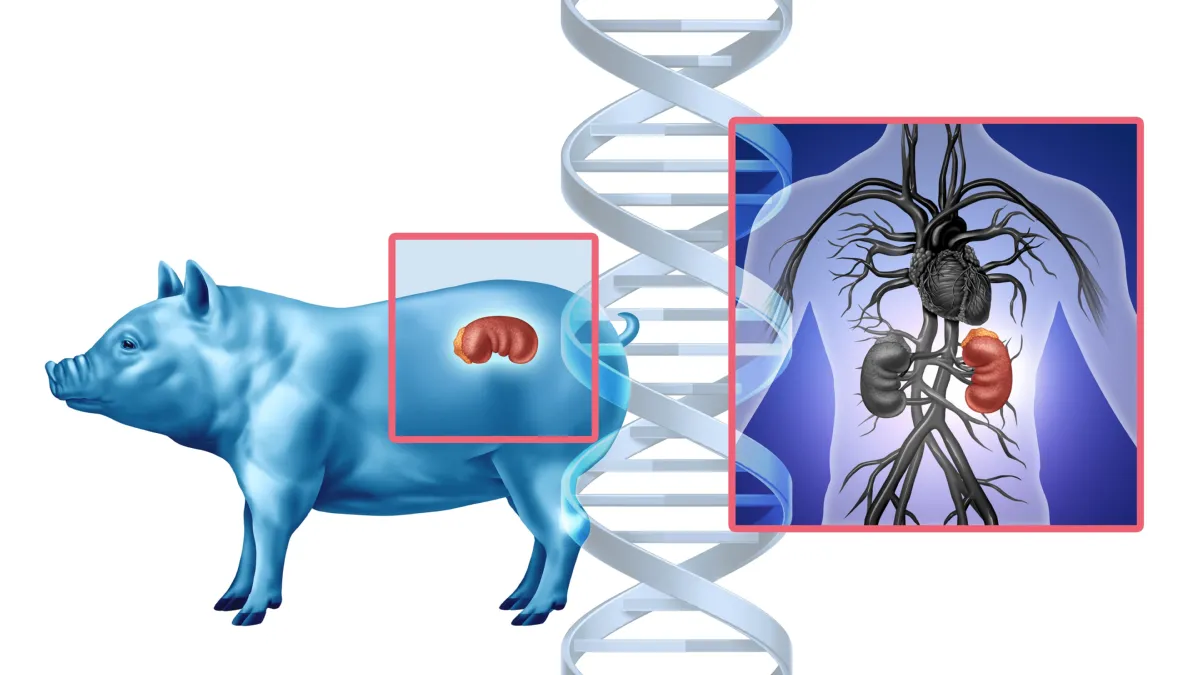
When a pig’s kidney is sewn into a human body, the human immune system doesn’t greet it with a handshake—it declares war.
This war, fought on a microscopic battlefield inside the body, has long stood in the way of one of medicine’s boldest dreams: xenotransplantation, or the transplanting of organs across species. But now, for the first time, scientists have opened a window into this hidden struggle, mapping the complex choreography of rejection and resistance in astonishing molecular detail.
Unveiled today at the ESOT Congress 2025, a pioneering study led by Dr. Valentin Goutaudier and an international team of researchers from the Paris Institute for Transplantation and Organ Regeneration and NYU Langone Transplant Institute offers unprecedented insight into what happens when a pig kidney is transplanted into a human. Their findings may well define the future of organ transplantation—and bring us closer to a world where no one dies waiting for a kidney.
A War in the Shadows: The Human Immune System vs. Pig Tissue
The human immune system is designed to protect us. It distinguishes between “self” and “non-self,” attacking anything that doesn’t belong—from bacteria to transplanted organs. In traditional human-to-human organ transplants, anti-rejection drugs are used to manage this response. But when the donor is a pig, even a genetically modified one, the immune system sees red.
This latest research captures that early reaction in remarkable detail.
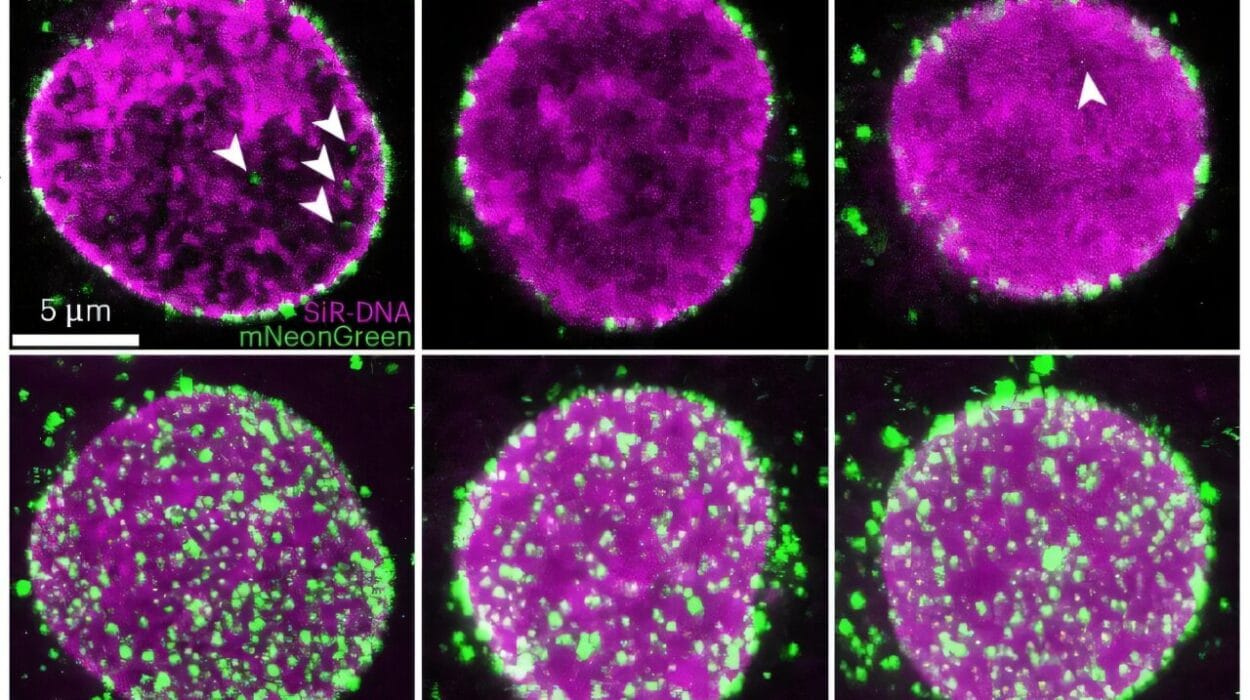
Using advanced spatial molecular imaging, the team watched—cell by cell, gene by gene—as human immune cells infiltrated every corner of the pig kidney’s filtering system. They didn’t merely graze its surface. They swarmed it. Like soldiers storming a foreign city, human immune cells established outposts in glomeruli, tubules, and vessels alike.
By Day 10 after transplantation, researchers could already see the molecular hallmarks of antibody-mediated rejection, the immune system’s high-powered offensive. By Day 33, the response peaked. Still, the most compelling revelation wasn’t just how quickly rejection began—but how it unfolded over time, offering a therapeutic window of opportunity.
Mapping the Invisible: Technology That Sees the Unseen
What sets this study apart is its exquisite resolution. Researchers deployed a bioinformatics pipeline capable of distinguishing human immune cells from pig kidney tissue—no easy feat when the two species’ cells are in physical contact, literally woven together in a living organ.
This enabled the team to create a spatial map of immune infiltration. The result? A vivid portrait of immune cell behavior over a full 61-day period.
Among the invading forces, macrophages and myeloid cells stood out as the most persistent and aggressive. These frontline immune cells aren’t new to transplant science—but their dominant role in cross-species rejection was laid bare here with more clarity than ever before.
“Our study provides the most detailed molecular map to date of how the human immune system engages with a transplanted pig kidney,” said Dr. Goutaudier. “By pinpointing specific immune cell behaviors and gene expressions, we can refine anti-rejection treatments and improve transplant viability.”
Turning the Tide: When Intervention Works
Armed with this map, the team tested targeted interventions. And they worked.
By introducing anti-rejection therapies at key points in the immune response timeline, they were able to reduce signs of tissue rejection. In other words, the immune system could be trained—or at least coaxed—into backing off.
This is more than just promising. It’s a crucial step toward making xenotransplants last. Even with genetically modified pigs designed to be more compatible with human biology, rejection remains the single greatest obstacle. Now, scientists have a better sense of when and how to intervene.
And timing, in the world of organ rejection, is everything.
The Bigger Picture: Solving a Global Crisis
Every year, hundreds of thousands of people around the world wait for organ transplants. Many never get one. Others die waiting. In the United States alone, over 90,000 people are on the kidney transplant waiting list. The shortage is not due to medical incapability—but supply.
Xenotransplantation could change that forever.
By engineering pigs whose organs are biologically compatible with humans, scientists could create a renewable, consistent source of organs. No more heartbreaking waits. No more dependence on the tragedy of donor death. It’s a vision that was once science fiction. Now, it’s inching closer to reality.
The first U.S.-based clinical trials of pig kidney transplantation into living human recipients are set to begin in 2025. The timing of this new study is not accidental—it comes as the world stands on the cusp of medical transformation.
What Comes Next: Engineering Tolerance
This study doesn’t just describe rejection. It suggests a path to tolerance.
With its molecular maps and detailed immune timelines, the research lays the groundwork for smarter drugs, personalized anti-rejection strategies, and improved genetic tweaks in donor pigs. It also points toward new early detection protocols—so physicians can act before rejection spirals out of control.
“Understanding the specific immune interactions at a molecular level allows us to develop targeted interventions that can prevent rejection before it escalates,” explained Dr. Goutaudier. “This research lays the groundwork for safer and more effective pig-to-human transplants in the near future.”
In short, this isn’t just about managing crisis. It’s about preventing it.
A Future Worth Fighting For
There is something deeply poetic in the idea that a pig’s organ—born in a barn, shaped by genetic science, and implanted in a human body—could become the lifeline for someone whose own organs have failed.
But poetry alone won’t make xenotransplantation real. It will take data, diligence, and a clear-eyed look at risk. These findings bring us a giant step closer.
The war between body and organ, self and other, won’t end overnight. But with each breakthrough—each study like this one—we draw the battle lines a little more clearly. And in doing so, we arm ourselves with knowledge powerful enough to save lives.
The message is clear: the future of organ transplantation isn’t waiting. It’s being built, molecule by molecule, cell by cell—in labs, in clinics, and, now, in the beating hearts of pigs whose lives may someday give others a second chance.