In the silent chambers of a pathology lab in Denmark, researchers have stumbled upon a revelation that may change how we think about one of humanity’s most insidious killers: atherosclerosis. This disease, known for clogging arteries and triggering heart attacks and strokes, has long been blamed on cholesterol, inflammation, and lifestyle. But a new study from the University of Southern Denmark and Odense University Hospital suggests a surprising twist—deep inside damaged arteries, the disease may be behaving more like cancer than we ever imagined.
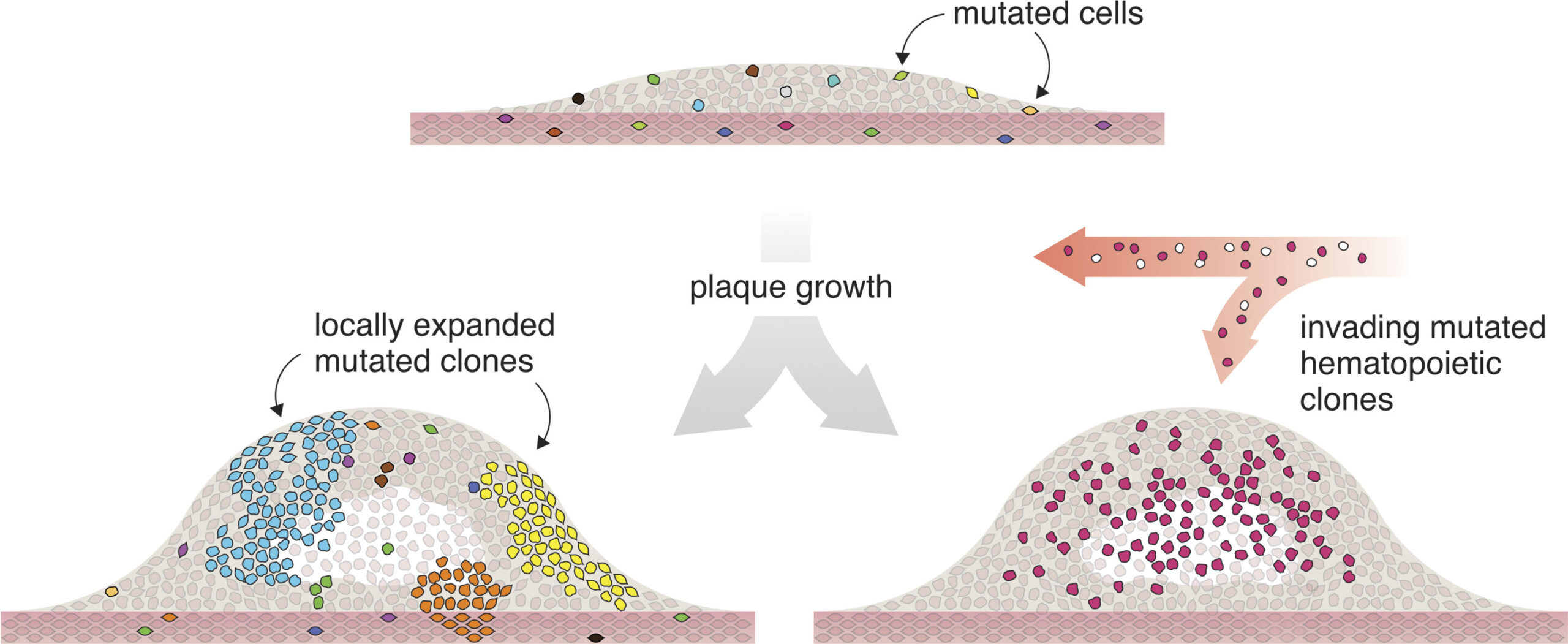
The team, led by Associate Professor Lasse Bach Steffensen, was analyzing tissue from patients who had undergone vascular surgery when they noticed something unusual. Many of the cells in the diseased blood vessel walls carried an identical genetic mutation—a signature typically seen in tumors. Even more astonishing, in some patients, more than 10% of the cells shared the same exact alteration, suggesting they were all descendants of a single rogue cell.
“It’s striking,” Steffensen said. “Hundreds of thousands of cells, all carrying the same genetic change. This kind of clonal expansion—where one mutated cell multiplies over and over—is something we associate with tumors, not with cardiovascular disease.”
The implication? Atherosclerosis, the disease behind many heart attacks and strokes, may have a hidden genetic engine—one that mimics the cell-division behavior of cancer.
A Familiar Disease, Seen in a New Light
For decades, atherosclerosis has been understood primarily through the lens of cholesterol buildup, immune responses, and arterial injury. Fatty deposits form plaques inside the vessel wall. These plaques grow silently for years, until one day they rupture, causing a sudden, catastrophic event—a heart attack, a stroke, or worse.
But what if part of this process is driven not just by fats and immune cells, but by a single mutated cell multiplying aggressively, quietly colonizing sections of the artery?
That’s what the Danish research team is beginning to suspect. Using advanced DNA sequencing, they compared the DNA of cells from diseased blood vessel tissue with the patients’ own blood cells. The comparison allowed them to isolate mutations that were unique to the diseased areas—genetic fingerprints of an unexpected kind.
What they found didn’t fit neatly into the established textbook picture.
“We’re not saying atherosclerosis is a blood vessel tumor,” Steffensen clarified. “But the biology we’re seeing—the presence of shared mutations in so many cells—definitely shares features with what we see in tumor growth.”
What Tumors Can Teach Us About Heart Disease
In cancer, a single cell undergoes a mutation that removes its normal checks and balances. It begins dividing, ignoring the usual rules, and its offspring inherit the same rebellious programming. Over time, a tumor emerges—a growing mass of identical cells.

In this new study, researchers observed a similar pattern in the arteries of people with atherosclerosis. A single mutation, a flood of identical daughter cells, and a growing mass—this time not of cancer, but of hardened, inflamed artery wall.
That insight is profound. It doesn’t mean atherosclerosis is cancer, but it suggests that the vessel wall may be far more dynamic—and far more genetically complex—than scientists had previously believed. The mutations weren’t random, either. Many occurred in genes linked to how cells behave: how they grow, move, or signal to each other.
“Some of these genes could influence how the cells respond to damage, or how they interact with cholesterol and inflammation,” said Steffensen. “That opens up new possibilities—not just for understanding the disease, but for how we might treat it.”
A Disease That Begins in Youth, Ends in Crisis
Atherosclerosis is a disease with a slow fuse. It begins young—often in adolescence—but gives little warning. Over decades, fatty cholesterol particles get trapped inside artery walls. The immune system, sensing an invader, sends inflammatory cells to the site. The resulting mess—fat, immune cells, and connective tissue—forms plaques that slowly narrow and stiffen the arteries.
At first, the body compensates. Blood finds new routes. But eventually, plaques grow too large or rupture, triggering clots that can block blood flow entirely. This is how many heart attacks and strokes happen—with little notice, and devastating results.
In Denmark, as in much of the world, atherosclerosis remains a leading cause of death. Current treatments focus on lowering cholesterol and blood pressure, reducing inflammation, and encouraging healthier lifestyles. But no therapies directly target the diseased vessel wall itself—especially not the rogue cells within it.
If further research confirms that clonal expansion of mutated cells plays a role in atherosclerosis, it could open new treatment frontiers. Scientists might one day target these specific cell lineages or prevent their abnormal growth altogether.
A Gift From Patients, Years in the Making
The breakthrough wouldn’t have been possible without the generosity of patients. The study was built on tissue samples collected over more than a decade from people undergoing vascular surgery at Odense University Hospital and Kolding Hospital. Each one voluntarily donated a piece of themselves—not for their own benefit, but to help push science forward.
Those samples were stored in a dedicated biobank, quietly accumulating until technology caught up. Using cutting-edge sequencing techniques, the researchers could now zoom in on the DNA of the vessel wall cells and find clues no microscope could ever reveal.
“This project is the product of years of collaboration—between surgeons, nurses, biochemists, and most importantly, the patients,” Steffensen said. “Without them, we wouldn’t have this glimpse into the disease’s genetic story.”
What’s Next: More Questions, Deeper Insights
While the findings are exciting, Steffensen is cautious. The study looked at only a small number of patients. Larger studies are underway, comparing tissue samples from more individuals to determine whether the same genetic patterns hold true across different stages of disease and different clinical backgrounds.
The team is also looking for patterns between mutation load and disease severity. Could people with more mutated clones be at higher risk of heart attacks? Could early detection of these cells be used to predict outcomes or guide treatment?
“We’re just scratching the surface,” Steffensen said. “But the more we look, the more we realize that this disease is more than just cholesterol and lifestyle. It’s also about cellular evolution—and how the body responds when a single cell starts rewriting the rules.”
A Shift in Perspective
The idea that a disease as common and well-studied as atherosclerosis might involve a hidden, cancer-like process is both unsettling and thrilling. It challenges long-held beliefs and invites a broader understanding of how complex our biology really is.
It also underscores a truth that medical science is learning again and again: diseases don’t fit into neat boxes. Cancer, heart disease, autoimmune conditions—more often than not, the boundaries blur. Our cells obey rules, but sometimes they bend them. And when they do, the consequences can be unexpected.
The arteries we once thought of as passive pipes may, in fact, be battlegrounds of microscopic competition and genetic drama—where a single cell’s quiet rebellion can echo through decades and end in heartbreak.
But knowledge is power. And the more we learn about the hidden layers of this disease, the closer we come to new ways of seeing, understanding, and eventually healing.
Reference: Lasse Bach Steffensen et al, Mutational landscape of atherosclerotic plaques reveals large clonal cell populations, JCI Insight (2025). DOI: 10.1172/jci.insight.188281