Ammonia is everywhere—working quietly behind the scenes of agriculture, pharmaceuticals, and countless industrial processes. Yet despite its usefulness, it carries a hidden danger. Ammonia (NH₃) is a colorless, pungent, and highly corrosive gas. When its concentration rises above 50 ppm, it can severely damage the lungs and, in extreme cases, become fatal.
The scale of exposure risk is staggering. More than 182 million tons of ammonia are produced every year through industrial and human activities, and roughly 80% of it eventually escapes into the atmosphere. This makes ammonia not only an industrial concern, but also a persistent environmental and public health challenge.
The greatest threat, however, is not just the gas itself—but the time it takes to detect it. Ammonia cannot be seen, and traditional sensors often respond too slowly to act as early warning systems. When a leak occurs, even a brief delay in detection can be dangerous.
Now, researchers from Guangxi University in China have built a sensor that responds in just 1.4 seconds—a speed that surpasses existing ammonia detection technologies. Their work, published in Nature Communications, reveals a design that borrows from the human body itself.
And at the heart of this invention lies an idea both simple and elegant: to detect a dangerous gas, first learn how to breathe like a lung.
Learning From the Quiet Architecture of Breathing
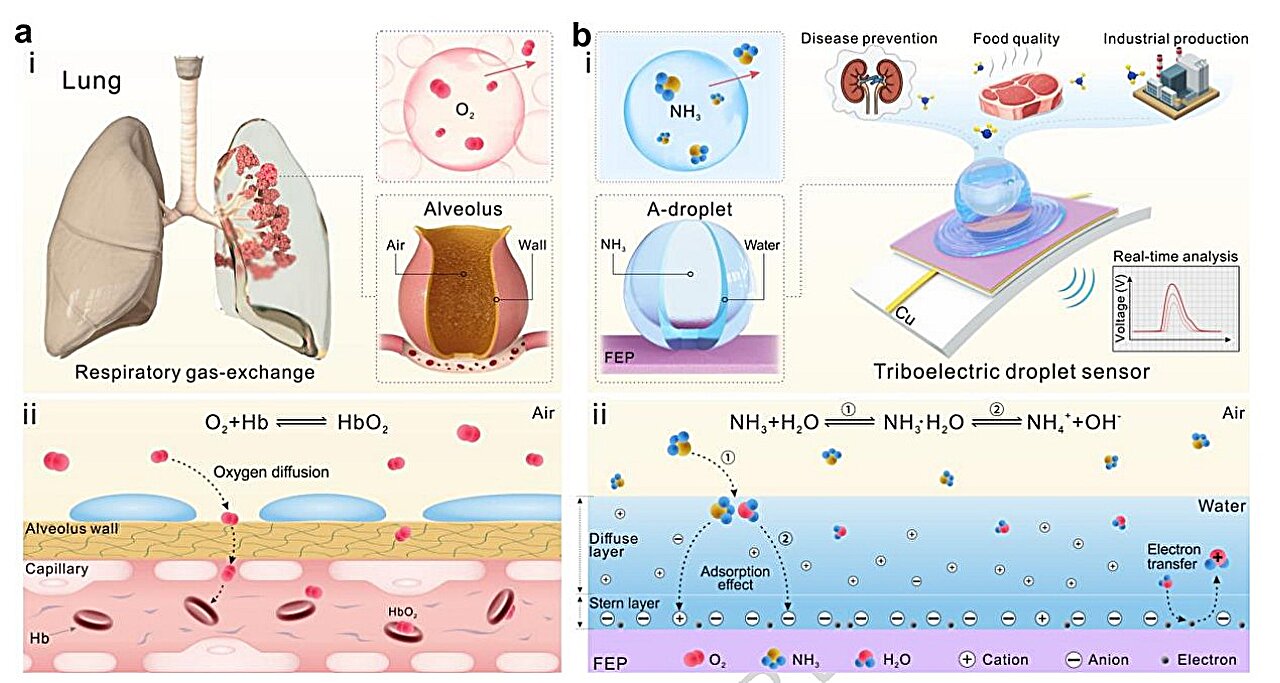
Deep inside the lungs are alveoli, tiny spherical air sacs that serve as the primary site of gas exchange. Each breath depends on them. Oxygen enters the bloodstream, carbon dioxide leaves, and this delicate exchange happens with remarkable efficiency.
The research team saw in these microscopic structures a blueprint for sensing gas with extraordinary speed. If alveoli can exchange gases almost instantly, perhaps a sensor designed in their likeness could capture ammonia just as quickly.
This inspiration gave rise to a unique detection mechanism built around A-droplets—tiny water droplets that contain a trapped air bubble. These droplets act as miniature gas exchangers, echoing the function of lung air sacs.
Ammonia has a strong affinity for water, meaning it readily dissolves when it encounters moisture. The A-droplets take advantage of this property. When ammonia is present, it rapidly diffuses into the droplets and dissolves. The process mirrors what happens in the lungs, where gases move quickly across thin surfaces into fluid environments.
In this sensor, however, the consequences of that interaction are not biological—they are electrical.
When a Falling Droplet Becomes a Signal
The device itself is powered by a triboelectric nanogenerator (TENG), a system that converts mechanical energy into electrical energy. Instead of requiring an external power supply, it generates electricity from motion—in this case, the impact of a droplet landing on its surface.
The sensor contains a dual-electrode copper setup. When an ammonia-carrying A-droplet falls and strikes the sensor, the impact completes an electrical circuit. That moment of contact produces a measurable electrical signal.
But the droplet is not just a trigger. It is also the messenger carrying chemical information.
When ammonia dissolves in the water of the droplet, it forms ammonium ions and hydroxide ions, making the droplet highly conductive. As the droplet spreads across the sensor surface, these ions interfere with the movement of electrons. This interference creates a shielding effect that reduces the electrical output.
The relationship is precise and revealing. Higher ammonia concentrations produce stronger shielding. Stronger shielding produces weaker electrical signals. By measuring how much the signal changes, the system can determine how much ammonia is present.
All of this happens incredibly fast. From droplet impact to concentration measurement, the process takes less than two seconds.
The Moment Electricity Becomes Meaning
Capturing electrical signals is only part of the story. To transform those signals into clear, reliable information, the researchers added another layer of intelligence.
The sensor converts raw electrical outputs into digital signals displayed in real time. These signals are then analyzed by an AI model that transforms them into time-frequency images—visual patterns representing how the signal changes over time and across frequencies.
The system was trained to recognize patterns associated with different ammonia levels. It learned to classify concentrations across five levels ranging from 0 to 200 ppm.
The result is not only rapid detection but remarkable precision. The system achieved up to 98.4% detection accuracy, distinguishing subtle differences in ammonia concentration with consistency and reliability.
In essence, the sensor does not simply detect ammonia—it interprets it, categorizes it, and communicates its presence almost instantly.
Why Speed Has Always Been the Missing Piece
Traditional ammonia sensors typically rely on solid-state detection. In these systems, gas molecules adhere to solid surfaces, form chemical bonds, and later detach. Only after this entire reaction cycle can a reading be generated.
This process takes time. And in situations where ammonia accumulates rapidly, even a short delay can be critical.
The new sensor avoids this limitation entirely. Instead of waiting for slow surface reactions, it uses droplets to capture ammonia instantly and converts mechanical motion directly into electrical signals. Detection becomes an immediate consequence of contact.
This fundamental shift—from waiting for chemical bonding to measuring dynamic electrical responses—removes the lag that has long limited ammonia monitoring technologies.
A Sensor That Moves Like Breath Itself
What makes this design especially striking is how seamlessly biology and engineering intertwine. The alveoli-inspired structure enables rapid gas absorption. The A-droplets function as moving exchange chambers. The triboelectric nanogenerator transforms motion into measurable energy. And AI-driven analysis turns raw signals into meaningful data.
Each element plays a role in mimicking a natural process while translating it into an electronic language. The system does not just detect gas—it behaves like a living mechanism designed for continuous sensing.
The entire sequence unfolds almost like a breath cycle. A droplet forms. It encounters gas. It absorbs, reacts, and signals. Then the process begins again.
Why This Research Matters
Ammonia is essential to modern industry, yet its risks are profound and widespread. With millions of tons entering the atmosphere each year, and exposure capable of causing severe lung damage above 50 ppm, rapid detection is not a convenience—it is a necessity.
The new bioinspired hydro-electrochemical sensor offers something previous technologies have struggled to achieve: speed without sacrificing accuracy. By delivering reliable measurements in just 1.4 seconds, it dramatically shortens the time between gas release and detection.
If adapted for real-world use, such rapid sensing could help prevent industrial accidents, reduce environmental exposure, and protect human health in settings where ammonia is produced, stored, or transported. Early detection means earlier response—and earlier response can mean the difference between safety and harm.
This research shows that sometimes the most advanced technological solutions emerge not from entirely new inventions, but from carefully observing how life itself solves similar problems. By learning from the lungs—the body’s own system for rapid gas exchange—scientists have created a sensor that reacts with almost biological urgency.
In a world where invisible dangers can spread silently, the ability to detect them in seconds is more than a technical achievement. It is a step toward making the air around us safer to breathe.
Study Details
Tao Liu et al, Bioinspired triboelectric droplet sensor for ammonia monitoring, Nature Communications (2026). DOI: 10.1038/s41467-026-68974-4