The periodic table may look like a simple grid of symbols and numbers, but it holds secrets deeper than any map of ancient treasure. Behind each element is a story—of electrons, attraction, force, and pattern. If you’ve ever wondered why some elements are explosive while others are noble, why fluorine is a furious thief and cesium practically throws electrons away, the answer lies in periodic trends.
These trends are the unspoken rules of the periodic table, the subtle shifts and flows of chemical behavior as you move across rows and down columns. To a beginner, these can seem abstract or overly technical. But with the right tricks and analogies, periodic trends reveal their secrets clearly and memorably.
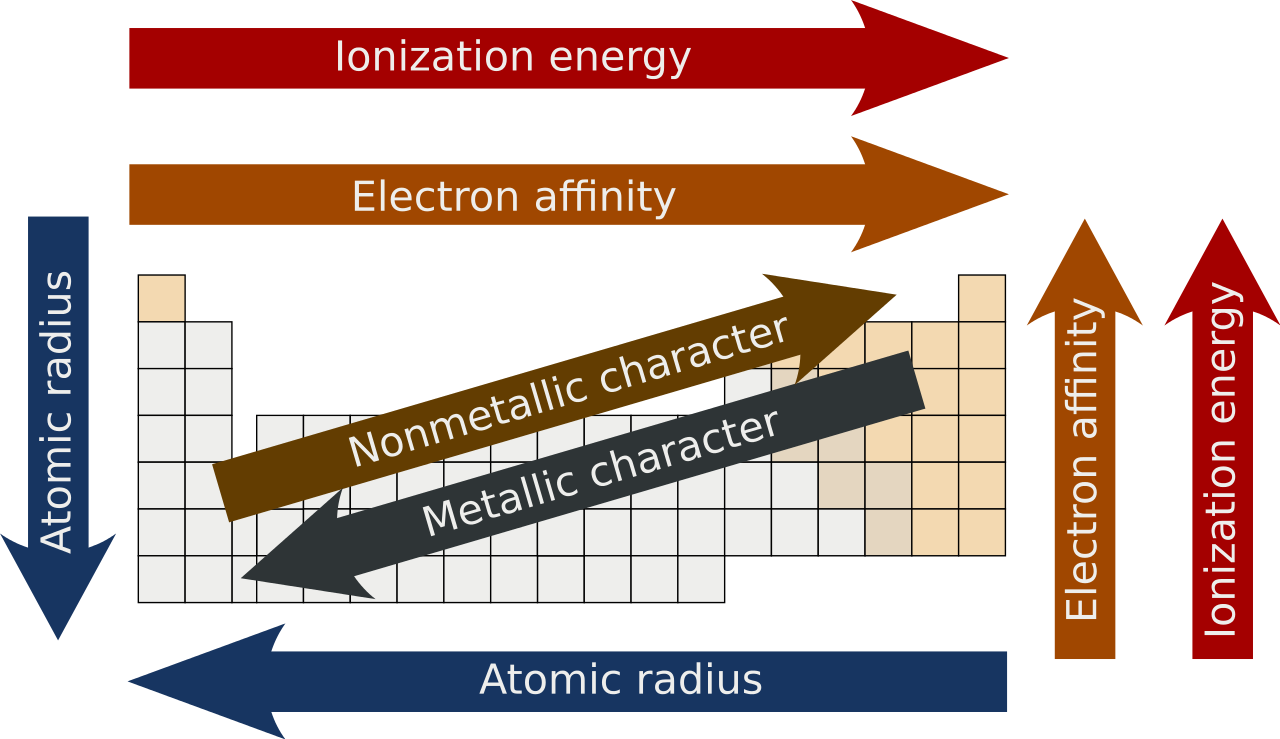
Let’s explore the core periodic trends—atomic radius, ionization energy, electronegativity, electron affinity, metallic character, and shielding effect—and break them down into simple, visual, and unforgettable lessons.
Atomic Radius: The Size Game of Atoms
Atomic radius refers to the size of an atom, from its nucleus to the outermost boundary where its electrons might be found. But atoms aren’t like billiard balls—they’re fuzzy, quantum clouds of probability. So when we say “size,” we’re talking about the average distance where an electron is likely to be found.
Here’s the simple trick: As you move left to right across a period, atoms shrink. As you move down a group, atoms grow.
Why? Moving across a period, you’re adding more protons to the nucleus and more electrons to the same energy level. The increasing positive charge in the nucleus pulls the electrons closer, like a tightening net. This “nuclear pull” makes the atom shrink.
But when you move down a group, you’re adding new electron shells—bigger orbits around the nucleus. These outer electrons are further away, making the atom larger, despite the greater nuclear charge.
Simple analogy: Think of a growing star with more gravity (protons). Across a row, the gravity increases but no new layers are added—so it collapses inward slightly. Down a column, you’re adding layers—like building a bigger onion—so size increases.
Ionization Energy: The Cost of Losing an Electron
Ionization energy is the energy required to remove an electron from an atom. It’s like prying a child’s favorite toy from their hands—the tighter they grip, the more effort it takes.
Ionization energy increases across a period (left to right) and decreases down a group.
Why does it increase across a period? Because atoms on the right side of the table (like fluorine and oxygen) desperately want to gain electrons, not lose them. Their electrons are held tightly by a strong nucleus. So taking an electron away from them is like trying to pull a dollar from a miser’s hand—very hard.
Why does it decrease down a group? Because those outer electrons are farther from the nucleus and shielded by inner electrons. They feel less pull and are more loosely held. So it’s like picking fruit from a tall, leafy tree—the further the fruit, the easier it is to pluck.
Trick to remember:
- Across a period: The atoms get smaller and hold their electrons tighter — so harder to remove electrons.
- Down a group: The atoms get bigger and the outer electrons are farther away — so easier to remove electrons.
Electronegativity: Who’s the Greediest?
Electronegativity is an atom’s ability to attract electrons in a chemical bond. It’s a popularity contest for electrons. The more electronegative an atom is, the more it pulls electrons toward itself.
Electronegativity increases across a period and decreases down a group.
Why? Across a period, atoms have more protons in their nucleus and a stronger pull on electrons. Plus, they are closer to having a full outer shell, which makes them more desperate to grab electrons. Fluorine, the most electronegative element, is just one electron away from a full set—it’s the prom queen of electron attraction.
Down a group, electronegativity decreases because the outer electrons are farther from the nucleus, and the nucleus’s pull is blocked by inner shells. These atoms become less effective at attracting bonding electrons.
Trick to remember:
- Fluorine is the most electronegative element. It sits at the top-right corner (excluding noble gases).
- Electronegativity is like hunger for electrons. Across a row: hunger increases. Down a column: they become sluggish and lazy.
Electron Affinity: The Desire to Gain an Electron
Electron affinity is the energy change when an atom gains an electron. You can think of it as the “joy” or “pain” an atom experiences when it receives a new electron.
Generally, electron affinity becomes more negative (more energy released) across a period and becomes less negative (or even positive) down a group.
This means that elements on the right side of the table (excluding noble gases) love gaining electrons and release lots of energy doing so. On the left, elements don’t care much—or might even resist—so less energy is released or even required.
Why the trend? As you go across a period, atoms are closer to filling their outer shell and welcome new electrons with open arms. But as you go down a group, added electrons are placed in orbitals farther from the nucleus and don’t experience as much attraction, so the energy release is smaller.
Simple trick: Think of the elements on the right side of the table (especially halogens) as electron lovers—they’re desperate for one more to fill their shell. The further you go down the group, the less passionate they become.
Metallic Character: How “Metal” an Element Is
Metallic character refers to how easily an atom loses electrons and behaves like a metal—shiny, conductive, and malleable.
Metallic character increases down a group and decreases across a period.
On the left side of the table, elements like sodium and potassium easily give up their electrons and are classic metals. On the right side, elements like chlorine and sulfur cling to their electrons and are nonmetals.
As you go down a group, the outer electrons are farther from the nucleus and more loosely held, so the atom gives them up more easily—this increases metallic character. As you go across a period, atoms hold on tighter to their electrons—this decreases metallic character.
Simple trick:
- Left and Down = More metallic
- Right and Up = Less metallic (more nonmetallic)
Shielding Effect: The Cloak Around the Nucleus
The shielding effect describes how inner electrons block the attraction between the nucleus and the outer (valence) electrons.
The more inner shells an atom has, the greater the shielding effect. This makes it easier for the atom to lose electrons and reduces the nucleus’s grip on outer electrons.
Shielding increases down a group, but stays relatively constant across a period.
That’s because within a period, you’re adding electrons to the same energy level—no new inner shells—so the shielding doesn’t change much. But as you go down a group, new inner shells are added, increasing the shielding.
Simple trick: Think of electrons as students in a classroom. The teacher is the nucleus. Inner electrons are students sitting up front, blocking the view. The more students in front (inner shells), the harder it is for the teacher to interact with the students in the back (valence electrons).
Why Noble Gases Are the Odd Ones Out
When discussing trends like electronegativity or electron affinity, noble gases often get left out. Why?
Because they already have full electron shells and don’t want to gain or lose electrons. They are the “satisfied” elements, chemically inert and happy in their atomic solitude.
So while electronegativity skyrockets across a period, noble gases at the far right don’t follow this pattern. Their electron affinity is often zero or even positive—meaning they don’t want more electrons. It’s like trying to sell more clothes to someone whose closet is already perfectly full.
Exceptions and Anomalies: The Universe Isn’t Perfect
Though the trends above generally hold true, the universe likes to throw curveballs. Some elements don’t follow the rules perfectly.
For example:
- Oxygen has a lower electron affinity than fluorine, but chlorine has a higher electron affinity than fluorine—even though it’s below it on the table.
- Some transition metals have irregular electron configurations due to the subtle interplay between s and d orbitals.
These exceptions arise due to the complex nature of electron repulsion, orbital energies, and subatomic interactions. They serve as a reminder that chemistry, while often predictable, is also rich with nuance.
Visualizing the Trends: Patterns in the Periodic Landscape
If you imagine the periodic table as a landscape, trends become easier to see:
- Atomic radius: like a shrinking spiral as you go right; a rising staircase as you go down.
- Ionization energy: climbs up a mountain from left to right; drops as you descend the column.
- Electronegativity: grows toward the top-right peak, with fluorine reigning as queen.
- Metallic character: a slide that descends as you go right; a lift that rises as you go down.
- Shielding effect: a blanket that thickens with each step down the table.
This mental imagery helps root the concepts in physical, spatial patterns, making them easier to recall and connect.
Real-Life Applications of Periodic Trends
Understanding periodic trends isn’t just for passing chemistry exams. These trends shape real-world phenomena in medicine, materials science, engineering, and even nutrition.
- Battery design depends on metals with low ionization energies (like lithium and sodium) to provide electrons easily.
- Corrosion resistance relates to an element’s reactivity, tied to ionization energy and electronegativity.
- Fertilizers and agriculture use elements like nitrogen, phosphorus, and potassium—chosen based on size, bonding behavior, and electron affinity.
- Pharmaceuticals rely on electronegative atoms (like oxygen and fluorine) to form stable and specific chemical interactions in the body.
The periodic table is not just a scientific tool—it’s a blueprint of how matter interacts, and periodic trends are its guiding principles.
A Memory Trick: The Trendy Table Tale
To help remember the trends, imagine a story:
Once upon a time, a small atom named Helio wanted to grow (atomic radius increases down). As he moved across the kingdom of Periodia, he shrank from the pressure of the royal nucleus. The tighter things got, the harder it became to escape (ionization energy rose), and the greedier the kingdom became for electrons (electronegativity rose).
But those in the lower lands grew larger and lazier, giving up their electrons without much fight (metallic character). They were well shielded from the king’s pull, cloaked by layers of loyal inner knights (shielding effect). Only those at the top-right tower—small, strong, and sharp—were the true electron kings.
And in the center of it all stood the noble ones, untouched and complete, watching the drama unfold with quiet dignity.
Conclusion: Mastering the Trends
The periodic table is more than a chart—it’s a narrative. Periodic trends are the grammar of the chemical language, the rules by which elements behave, interact, and react. By understanding these trends, chemistry becomes less about memorization and more about intuition.
From the shrinking atoms of the right side to the generous metals of the left, from the top-tier electron hoarders to the bottom-layered giants, periodic trends tell a story of balance, attraction, and transformation.
The key is to internalize the patterns, not just recite them. Use analogies, visual landscapes, and little memory tales to anchor the ideas. Because once you truly see the trends, the periodic table stops being a chart—and starts becoming a map of the universe.