In a quiet lab where biotechnology and immunology collide, researchers may have taken a major step toward one of medicine’s most elusive goals: a single-shot vaccine for HIV. It’s a disease that has defied decades of scientific effort, evolving like a ghost through the immune system, always a step ahead of the body’s defenses.
But now, scientists from MIT and the Scripps Research Institute have found a way to give the immune system a stronger weapon—and they’ve done it with a powerful blend of materials called adjuvants, which stimulate the body’s natural defenses. By combining two such adjuvants, the researchers have created a vaccine that—at least in mice—generates a wider, deeper, and longer-lasting immune response than traditional vaccines.
The results, published this week in Science Translational Medicine, suggest something remarkable: one dose could be enough to build robust protection, not just against HIV, but possibly against other fast-mutating pathogens like influenza and SARS-CoV-2.
“The immune system is like a brilliant but distracted student,” said Dr. J. Christopher Love, senior author and MIT professor of chemical engineering. “Our goal was to help it focus—and remember—for as long as possible.”
Two Adjuvants Are Better Than One
Vaccines rely on teaching the immune system to recognize a viral threat before it strikes. But that lesson doesn’t always stick—especially when the virus, like HIV, constantly changes its disguise. To help the immune system “remember” the right parts of the virus, scientists often include adjuvants—immune-stimulating agents that give the body a helpful nudge.
One of the most common is aluminum hydroxide, known as alum, used for decades in hepatitis and other vaccines. Another is saponin, a natural molecule derived from the Chilean soapbark tree, which has shown great promise when combined with other immune activators.
Dr. Darrell Irvine of Scripps had previously designed a new version of saponin packaged in tiny particles along with MPLA, a molecule that promotes inflammation. These particles, known as SMNP, are already being tested in clinical trials as part of experimental HIV vaccines.
So Love and Irvine asked: What happens if we combine alum and SMNP?
The answer, it turns out, is that the immune system doesn’t just get a nudge—it gets a full course of memory training.
A Vaccine That Lingers Where It Matters
The team engineered their vaccine by attaching dozens of HIV antigens to alum particles and combining them with SMNP. When they injected the mixture into mice, the vaccine didn’t just vanish into the bloodstream. Instead, it accumulated in the lymph nodes—the immune system’s control rooms—and stayed there.
Not for hours. For up to a month.
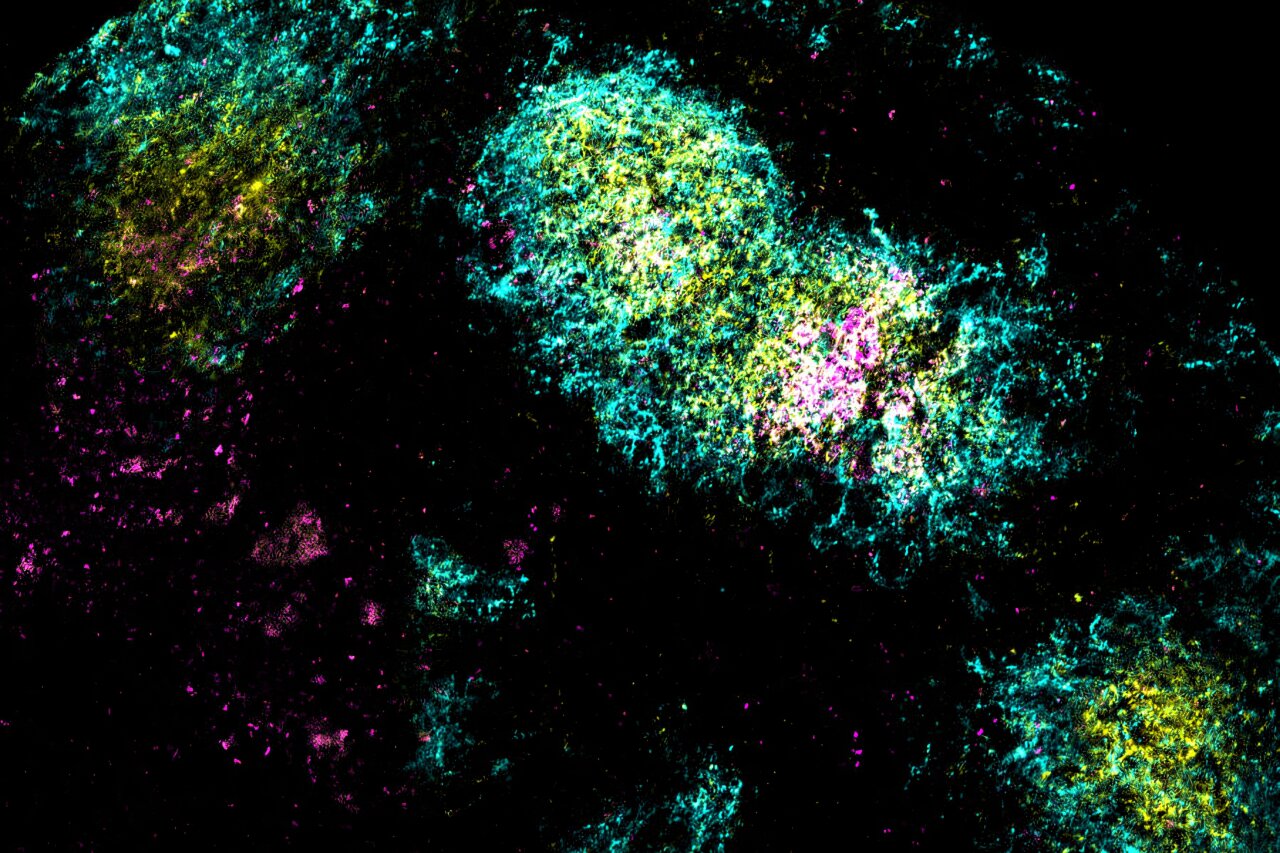
During that time, B cells—immune cells responsible for making antibodies—engaged in a furious cycle of testing and mutation inside germinal centers, the heart of immune training. With antigens still present weeks after vaccination, the B cells had time to evolve, refining their ability to recognize the HIV protein with exquisite precision.
“This process mimics what happens in a real infection,” Love explained. “The antigen sticks around, giving the immune system repeated exposure. It’s like a masterclass in viral recognition.”
This long-term antigen exposure is rarely achieved with standard vaccines, where the immune system might see the target protein only briefly before it degrades. But with this dual-adjuvant approach, the HIV protein was preserved and presented over a far longer period—just as it might be during a slow-moving infection.
A Symphony of B Cells
The most impressive result came when the team looked not just at the quantity of the immune response, but its quality.
Using single-cell RNA sequencing, a cutting-edge method for analyzing individual immune cells, the researchers compared B cells from mice given the dual-adjuvant vaccine versus those given only one adjuvant.
The difference was dramatic. The mice that received both alum and SMNP produced two to three times more unique B cells, each one generating a slightly different antibody. That diversity is crucial in the fight against HIV, a virus notorious for its genetic variability.
“Think of it like a lock-picking team,” said lead author Dr. Kristen Rodrigues. “If you only have one or two picks, you might miss. But if you have hundreds of different shapes, you’re much more likely to find the right fit.”
This broadened immune response improves the chance of creating broadly neutralizing antibodies—the kind that can recognize many strains of a virus, not just the one included in the vaccine.
Hope Beyond HIV
While the study focused on HIV, the implications stretch far wider. The same approach—stabilizing the antigen in the lymph nodes and maximizing immune training—could work for other challenging viruses, including SARS-CoV-2 and influenza, both of which mutate quickly and require regular vaccine updates.
Even more tantalizing is the possibility of creating single-dose vaccines, eliminating the need for repeated boosters.
“It’s not about reinventing the vaccine,” said Dr. Yiming Zhang, co-lead author. “It’s about re-engineering how we deliver it to the immune system—how we speak its language more clearly.”
Because both adjuvants used in the study—alum and SMNP—are based on well-characterized, FDA-approved components, the path to clinical trials could be shorter than for entirely new technologies. The challenge ahead lies in translating these mouse results to humans and confirming that the prolonged lymph node exposure and antibody diversity occur the same way in people.
The Future of Vaccine Design
This work opens a new chapter in vaccine science—one where the immune system isn’t just shown an enemy, but is given the tools, time, and training to become a master strategist. It’s not about making louder alarms, but smarter instructions.
It’s a shift from brute-force stimulation to precision tutoring.
The researchers are already exploring how this dual-adjuvant strategy could be applied to next-generation COVID-19 vaccines, and possibly even cancer vaccines, where training the immune system to recognize subtle, personalized targets is the holy grail.
A New Hope for Hard-to-Vaccinate Diseases
HIV has long been a shadow in the world of infectious disease—a moving target, impossible to pin down. But this study offers a glimmer of light, not just for HIV, but for our entire approach to vaccine design.
In a world where pandemics emerge with terrifying speed, the ability to create potent, long-lasting immunity in a single dose could be a game-changer.
And the secret, it seems, might lie not in the antigen itself—but in how it’s delivered, and for how long it stays.
Reference: Kristen A. Rodrigues et al, Vaccines combining slow release and follicle targeting of antigens increase germinal center B cell diversity and clonal expansion, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adw7499