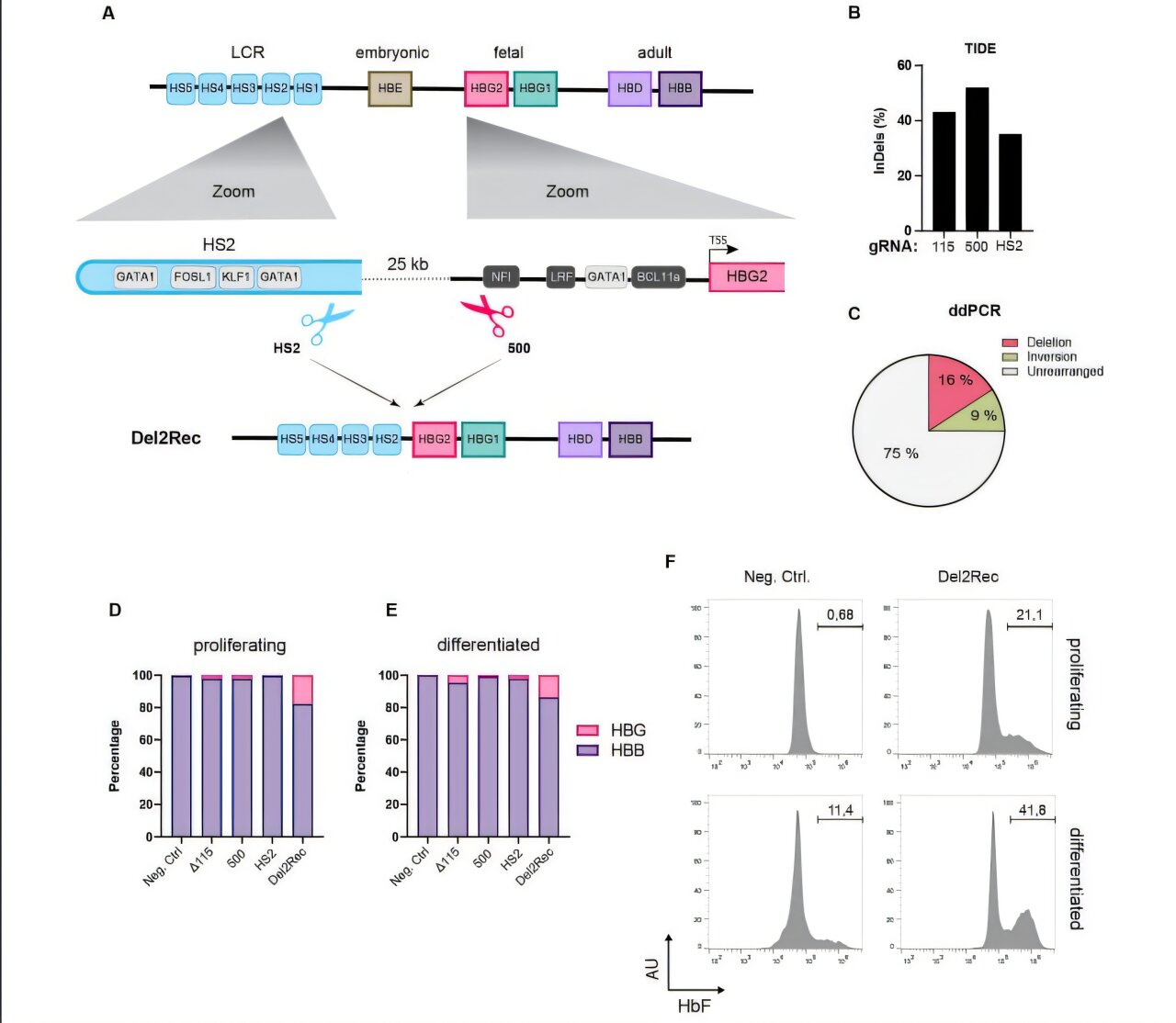
In a quiet laboratory in the Netherlands, scientists have made a bold leap toward rewriting the future of gene therapy—not by inserting new genes, but by rearranging nature’s existing script. Using the gene-editing tool CRISPR-Cas9, a Dutch research team has successfully reactivated dormant genes by altering their proximity to genetic switches known as enhancers. The method, dubbed “delete-to-recruit,” offers a radically simple yet powerful new strategy to treat genetic diseases like sickle cell disease and beta-thalassemia.
This approach doesn’t add foreign genetic material. It doesn’t tweak the core code of the gene itself. Instead, it moves pieces of DNA around—bringing broken systems closer to their forgotten backups, and letting the body do the rest.
A New Way to Turn Genes Back On
Imagine an old backup generator gathering dust in your basement. The power grid fails, and your house goes dark. You don’t need to build a new engine—you just need to flip the switch and bring that backup to life.
That’s essentially what the researchers at the Hubrecht Institute, Erasmus MC, and Sanquin have done, using CRISPR-Cas9 to cut out a stretch of DNA that separates an enhancer (the switch) from a normally silent gene (the generator). By deleting that intervening DNA, they moved the enhancer closer, giving it the power to turn on a gene that would otherwise remain silent.
“In adult cells, this successfully reactivated genes that are normally only active during embryonic development,” explained Anna-Karina Felder, one of the study’s lead authors. “It’s like rewiring the circuit to bring light where there was none.”
The implications are profound—not just for our understanding of gene regulation, but for real patients battling life-threatening blood disorders.
Fighting Genetic Blood Diseases with Nature’s Own Tools
Sickle cell disease and beta-thalassemia are caused by defective adult globin genes. These genes are responsible for producing the hemoglobin that carries oxygen in red blood cells. When they malfunction, red blood cells become misshapen or unstable, leading to symptoms like chronic fatigue, organ damage, and a lifetime of transfusions.
But humans aren’t born with broken hemoglobin. In the womb, a different gene—a fetal globin gene—takes the lead. It powers red blood cell production during development, ensuring oxygen delivery before birth. Then, just after we enter the world, that gene is quietly switched off as the adult version takes over.
In patients with these blood diseases, that switch is tragically mistimed. The adult gene fails—but the fetal gene remains intact, simply lying dormant.
Enter delete-to-recruit.
“In people with sickle cell disease or beta-thalassemia, it’s the adult globin gene—the main engine—that’s broken,” Felder said. “But fetal globin is like a backup engine. By switching it back on, we can repower the red blood cells and possibly cure these patients.”
Precision Without Invasion
What makes this technique so remarkable is its simplicity and precision. Traditional gene therapies often involve adding entirely new genes or disabling repressors that silence helpful ones—actions that risk off-target effects and long-term unpredictability. They’re also notoriously expensive and difficult to deliver.
The delete-to-recruit method is different.
It doesn’t modify the gene itself. It doesn’t introduce foreign elements. Instead, it leverages the architecture of the genome, cutting out the unneeded segments that separate an enhancer from a gene it could help.
Using CRISPR-Cas9 as molecular scissors, the researchers deleted the DNA “spacer” between an enhancer and the fetal globin gene. Once the distance was gone, the enhancer reached across and turned the gene on.
No foreign DNA. No artificial promoters. Just smart editing of what’s already there.
From Petri Dishes to Patient Cells
The team didn’t stop with simulations or isolated cells. They collaborated with experts from Erasmus MC (Philipsen group) and Sanquin (Van den Akker group) to test their method in real human cells—including cells from patients with sickle cell disease.
Most importantly, they targeted blood stem cells—the master cells that generate all types of blood cells in the body. These are the critical targets for any long-term treatment of blood disorders.
And the results were promising. In these modified stem cells, the fetal globin gene was reactivated, giving rise to red blood cells that produced functional hemoglobin—potentially bypassing the broken adult globin entirely.
A Blueprint for Broader Applications
While the immediate spotlight is on sickle cell disease and beta-thalassemia, the implications of this research extend far beyond blood.
Many diseases arise not from the complete absence of a gene, but from the failure of one version while another, often fetal or tissue-specific, version lies dormant nearby. Delete-to-recruit could be a way to tap into these silent reserves—reactivating alternative genes to fill in when the primary ones fail.
“This research lays important groundwork for the development of a whole new class of gene therapies,” Felder said. “And it could apply to many diseases where turning on a dormant gene might compensate for a faulty one.”
That’s not just an incremental step—it’s a paradigm shift.
A Gentler, More Affordable Gene Therapy
In 2024, Europe approved a gene therapy for sickle cell disease that modifies a globin repressor gene to reactivate fetal globin. But that approach is costly and complex. It can also lead to unintended effects on other genes, since repressors often regulate multiple targets.
Delete-to-recruit offers a gentler path. By focusing on physical proximity between enhancers and target genes, it avoids tampering with complex regulatory systems. It’s elegant, intuitive, and may avoid some of the risks and costs of more invasive gene-editing techniques.
Of course, the research is still in its early days. The experiments have been done in cell cultures, and clinical trials are still on the horizon. But the roadmap is clearer than ever—and the destination could be life-changing for millions of people.
Rewriting the Script of Genetic Disease
There is something poetic in the approach: not fixing what’s broken, not adding what’s foreign, but simply reconnecting the right parts of a story that already exists.
In the grand narrative of human biology, every gene is a character, and every enhancer a narrator. Sometimes, disease is just a matter of miscommunication—of the right story being told to the wrong chapter.
The Dutch researchers have found a way to turn the page.
As Felder put it: “We’re not introducing something new. We’re just helping the genome remember what it once knew how to do.”
And in that act of remembering, we may discover a new future for gene therapy—one where healing comes not from rewriting the code of life, but from reconnecting the sentences already written.
Reference: Anna-Karina Felder et al, Reactivation of developmentally silenced globin genes through forced linear recruitment of remote enhancers, Blood Journal (2025). DOI: 10.1182/blood.2024028128