Scientific progress rarely moves in straight lines. Sometimes, it leaps forward from the most unexpected places—like a cancer study that may now hold the key to treating a completely different disease: type 1 diabetes.
At the Mayo Clinic, a team of immunologists exploring how cancer cells evade immune detection made a surprising discovery. They identified a sugar molecule that helps cancer cells “hide” from the immune system. In a groundbreaking twist, they found that the same sugar molecule, when applied to insulin-producing cells, may help protect them from autoimmune attack—the hallmark of type 1 diabetes.
“It’s not often you can flip a mechanism like this from one disease to another,” says Dr. Virginia Shapiro, the principal investigator of the study. “But that’s exactly what we’ve done.”
Published in the Journal of Clinical Investigation, the findings represent an exciting step forward in diabetes research and offer a glimpse of a future where we might shield healthy cells from immune attack—without suppressing the entire immune system.
Understanding Type 1 Diabetes: When the Body Turns Against Itself
Type 1 diabetes is a lifelong autoimmune disorder in which the body’s immune system mistakenly destroys its own insulin-producing beta cells in the pancreas. Without insulin, the body cannot regulate blood sugar, leading to dangerous highs and lows that must be controlled through lifelong insulin therapy.
The disease affects around 1.3 million people in the U.S. alone, including children and young adults. Unlike type 2 diabetes, which is linked to lifestyle and metabolic factors, type 1 diabetes is triggered by a misfiring immune response—one that sees the body’s own cells as invaders and wages war on them.
Current treatments rely heavily on insulin injections or, in advanced cases, transplantation of pancreatic islet cells. But transplants come with a heavy cost: the need for drugs that suppress the immune system broadly, leaving patients vulnerable to infections and other complications.
The Mayo Clinic researchers were determined to find a better way—one that would prevent the immune system from attacking beta cells without compromising the body’s broader ability to fight disease.
From Tumor Trickery to Immune Tolerance
Dr. Shapiro and her team had already made waves in the field of cancer immunology by studying how tumors outwit the immune system. One key tool in the tumor’s arsenal is a sugar molecule called sialic acid. This molecule cloaks the surface of cancer cells, making them appear harmless to immune surveillance systems.
The cancer cells don’t produce sialic acid by themselves—they rely on an enzyme called ST8Sia6, which decorates their surfaces with this molecular camouflage. The team’s lightbulb moment came when they asked a radical question: What if we could use this same mechanism not to protect cancer cells, but to protect healthy ones from autoimmune attack?
It was a daring idea. Cancer cells use this strategy to deceive the immune system. Could the very same approach be harnessed in a constructive way—to teach the immune system not to attack beta cells in type 1 diabetes?
The Preclinical Proof: Sugar-Coated Beta Cells That Survive
To test the concept, the team first worked with an artificial model of diabetes. The results were encouraging. But to get closer to real-world disease, they moved on to a preclinical model that naturally develops autoimmune type 1 diabetes—a better approximation of what happens in human patients.
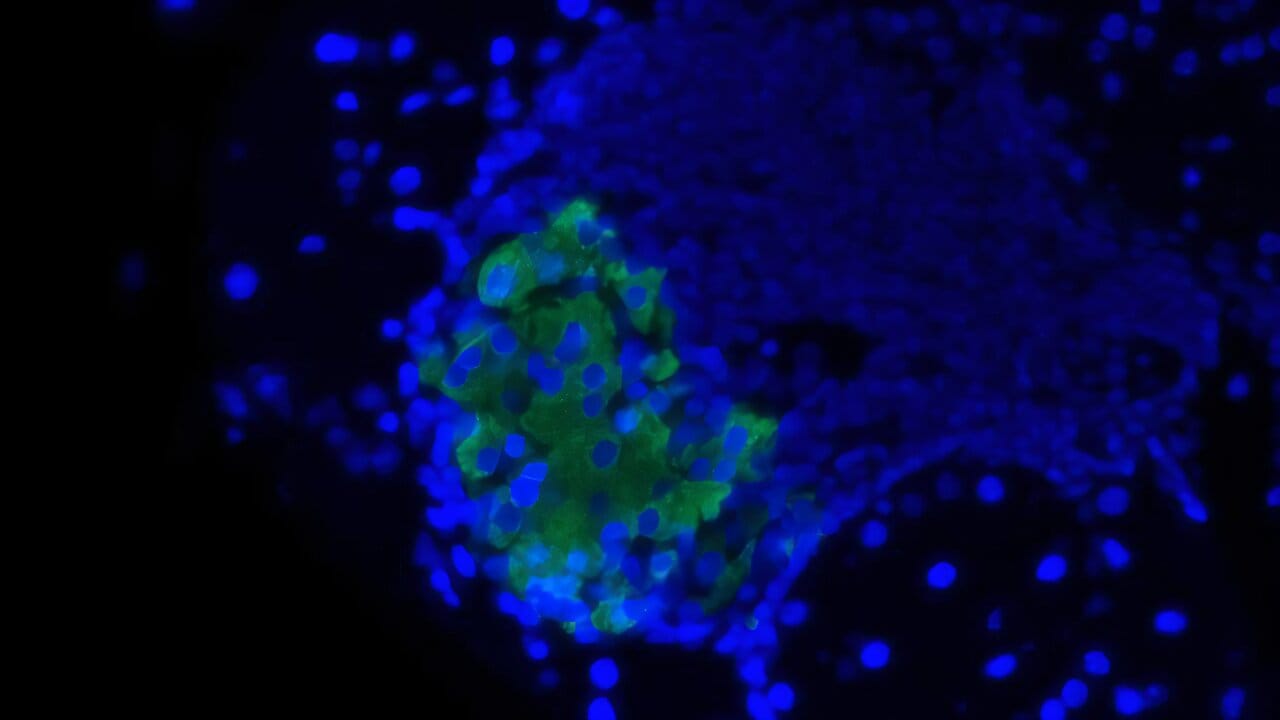
They engineered the beta cells in these models to express the ST8Sia6 enzyme, effectively sugar-coating themselves with sialic acid. The results were astonishing: in 90% of cases, the immune system did not attack the treated beta cells. The very cells that are normally destroyed in the early stages of type 1 diabetes were now preserved.
Dr. Shapiro’s team had achieved something remarkable: beta cells that looked “normal” to the immune system, despite the autoimmune context.
A Targeted Defense Without Shutting Down Immunity
One of the most compelling aspects of the study was not just the preservation of beta cells, but how specific that protection was.
“Though the beta cells were spared, the immune system remained intact,” explains Justin Choe, M.D.-Ph.D. student and lead author of the study. “We still saw active immune responses elsewhere in the body—against unrelated disease models. The engineered protection didn’t disable the immune system. It focused it.”
That specificity is a game-changer. Most current strategies to prevent autoimmune attack involve systemic immunosuppression, which makes the patient vulnerable to infections, cancer, and other diseases. But this approach appears to be local and selective—it turns down the immune attack on beta cells, while leaving the rest of the immune system fully operational.
If this strategy holds up in further testing, it could offer a path toward safer, more effective therapies—not just for diabetes, but for other autoimmune conditions as well.
The Road Ahead: Toward Cell Transplants Without Immunosuppression
While still in the experimental stage, the Mayo team’s findings have already opened a new avenue for research and potential therapies.
One major application may be in the field of islet cell transplantation, where beta cells from donors are implanted into patients with type 1 diabetes. Currently, these transplants require patients to take immune-suppressing drugs for life. But what if we could transplant engineered beta cells that the body doesn’t reject?
“That’s the dream,” says Dr. Shapiro. “To provide transplantable beta cells that don’t require immunosuppression. If we can do that, we could change the way we treat type 1 diabetes.”
Her lab is now working on integrating this approach with islet transplantation, with hopes of bringing the strategy closer to clinical trials in humans.
Implications Beyond Diabetes
While the study focused specifically on type 1 diabetes, its broader implications are hard to ignore. Autoimmunity is a central feature in diseases ranging from multiple sclerosis to lupus to rheumatoid arthritis. If we can engineer cells to avoid unwanted immune attack—without suppressing immunity as a whole—we could be on the brink of a new class of precision immunotherapies.
The research also reminds us of the deeply interconnected nature of biology. The same sugar molecule that shields tumors from destruction may one day help patients with diabetes live without insulin pumps or injections. What began as a tool of disease might soon become an instrument of healing.
Hope with Humility: The Human Side of Science
For all the excitement, the Mayo team is clear-eyed about the challenges ahead. Preclinical success does not always translate to humans. The complexity of human immune systems, the variability between patients, and the intricacies of beta cell biology all stand as hurdles.
But for families living with type 1 diabetes, even the possibility of a therapy that preserves beta cells—and reduces dependence on insulin—is a reason for hope.
“We’re still early in the process,” Dr. Shapiro says. “But studies like this remind us that sometimes, when you look at a problem from a new angle, you find answers you didn’t know were there.”
The next steps will involve rigorous testing, safety trials, and eventually, if all goes well, clinical trials in humans. But the spark has been lit.
Science, like life, doesn’t always go where we expect. Sometimes, it takes a winding path—from cancer to diabetes, from defense to healing, from the worst of nature to the best of human ingenuity.
More information: ST8Sia6 overexpression protects pancreatic β cells from spontaneous autoimmune diabetes in nonobese diabetic mice, Journal of Clinical Investigation (2025). DOI: 10.1172/JCI181207 , www.jci.org/articles/view/181207