In a quiet lab nestled in the heart of the University of Wisconsin–Madison, a remarkable discovery is breathing new hope into one of medicine’s fiercest battles—keeping aggressive cancers from coming back.
Harnessing the final moments of a dying cancer cell, scientists have crafted a revolutionary kind of personalized cancer vaccine—one that quite literally builds a new line of defense from the ashes of the disease itself. It’s a scientific sleight of hand that, if successful in humans, could transform the way we fight recurrent tumors.
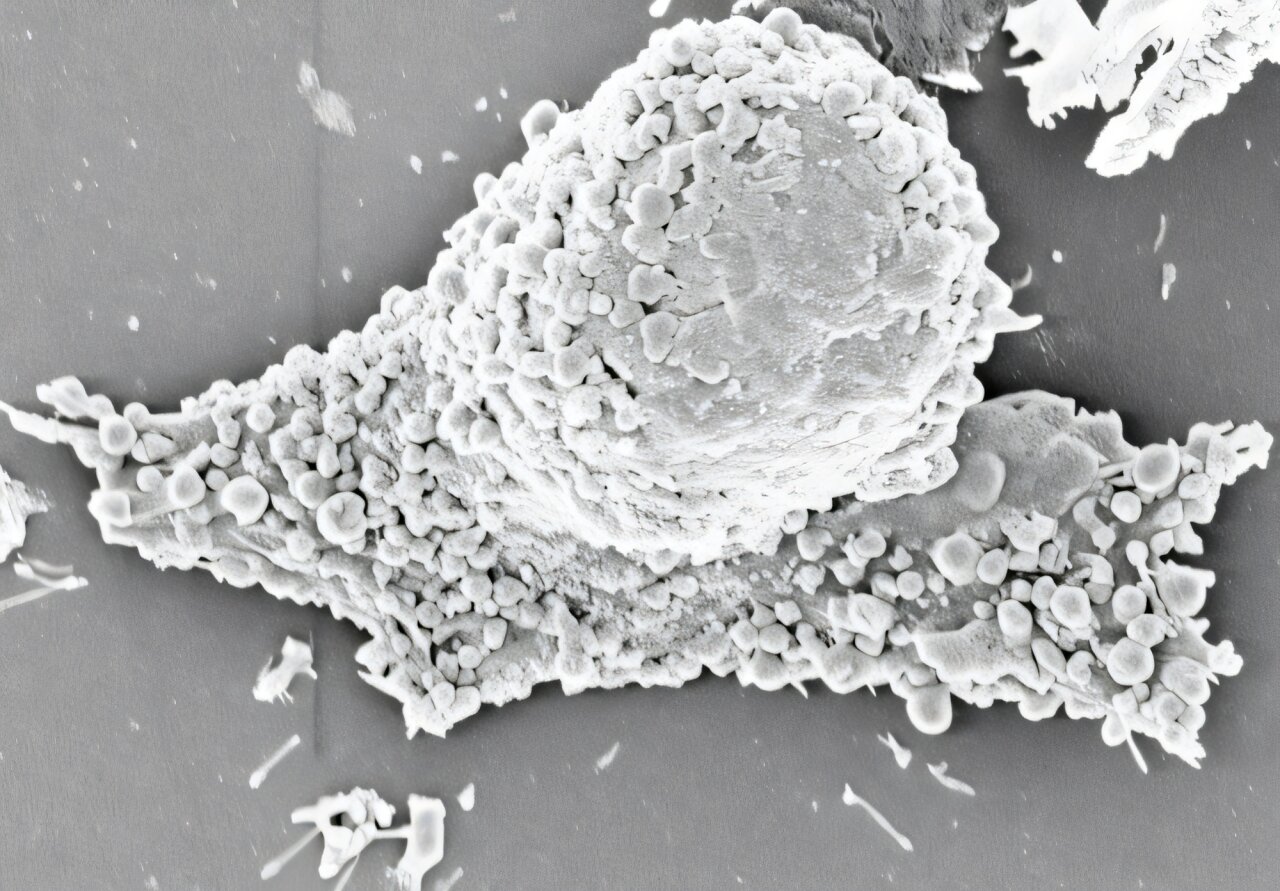
The research, led by Professor Quanyin Hu from the UW–Madison School of Pharmacy, is centered around an unexpected byproduct of cancer treatment: microscopic bubbles called pyroptotic vesicles. These tiny sacs emerge as a form of cellular debris during pyroptosis, a highly inflammatory type of programmed cell death. But what was once considered biological waste may hold the key to an entirely new class of cancer immunotherapy.
The Secret Weapon Hidden in Cellular Debris
“Compared to the other approaches, ours shows a much stronger immune response,” Hu said in an interview. His voice, while measured, carries the excitement of someone standing on the edge of something vast.
The breakthrough is rooted in a deceptively simple idea: When a cancer cell dies, it doesn’t just disappear. It leaves behind molecular fingerprints—fragments of DNA, proteins, and especially tumor-specific antigens, which are the molecular identity cards of cancer cells. Pyroptotic vesicles are essentially nature’s little envelopes, packed full of these fragments.
“These vesicles are like postcards from the battlefield,” explains Hu. “They carry vivid molecular memories of the enemy we’re fighting.”
What makes these vesicles extraordinary is that they are custom-built by the tumor itself. Each vesicle is specific to the cancer from which it came, containing the exact molecular markers needed to train the immune system to recognize and attack any stragglers.
In their newly published study in Nature Nanotechnology, Hu’s team engineered these vesicles to deliver a double punch. First, they are loaded with an immune-stimulating drug to activate and amplify the immune response. Then, they’re embedded in a hydrogel implant—a soft, jelly-like substance that is placed directly in the void left behind after a tumor is surgically removed.
It’s here, in the body’s newly scarred tissue, that the vaccine does its work. The hydrogel slowly releases the vesicles, teaching the immune system to seek out any remaining cancer cells and destroy them before they can regroup.
Turning a Corner in the Fight Against Recurrent Cancer
Cancer’s most fearsome weapon is not just its initial attack—it’s the ability to return. Triple-negative breast cancer, melanoma, and glioblastoma, among others, have especially high recurrence rates. Even after surgery, chemotherapy, and radiation, microscopic cancer cells can hide in tissues, waiting silently to reignite the disease.
“This is where our approach stands apart,” Hu explains. “We’re not just removing the tumor. We’re leaving behind a miniature training camp for the immune system, right where the enemy once lived.”
The results in mice are nothing short of stunning. In several models of triple-negative breast cancer—including one using human-derived tumors—and in a mouse model of melanoma, the hydrogel vaccine dramatically extended survival. In some cases, the mice remained cancer-free for the duration of the study.
“Essentially, we cured them,” Hu says. “No recurrence. That’s what makes this so promising.”
From Lab Bench to Clinical Bedside
Of course, between promise and proof lies a long road of rigorous testing. So far, the vaccine has only been tested in mouse models. The next step will be more trials in other animal models before human clinical trials can even be considered.
But the potential is staggering.
Unlike many current cancer vaccines—which must be administered systemically, often triggering wide-ranging side effects—this vaccine is localized. It is applied directly to the surgical site. This method not only focuses the immune response where it’s needed most, but dramatically reduces the risk of adverse effects elsewhere in the body.
“It’s a more elegant solution,” Hu says. “We’re leveraging the immune system, but doing it in a way that’s more targeted, more personal, and hopefully, more effective.”
A Personalized War Plan Against Cancer
Perhaps the most powerful aspect of the new approach is its personalization. Because the vesicles are derived from a patient’s own tumor, each vaccine is uniquely tailored. It’s a kind of biological fingerprinting—turning the cancer’s own identity into its downfall.
This personalization could be especially useful in treating cancers notorious for recurrence and resistance to standard therapies. Hu’s team is already eyeing applications in pancreatic cancer and glioblastoma, two of the deadliest and most difficult cancers to treat.
These cancers are cunning. They evolve rapidly, hide effectively, and often resist even the most aggressive treatment regimens. But with a vaccine made from the tumor itself, the immune system gets a head start—an insider’s look at what the enemy looks like before the next attack.
Looking Beyond the Horizon
For now, the research remains in the preclinical phase. But its implications are capturing the imagination of oncologists and immunologists alike. If successful in human trials, this method could reshape how we think about post-surgical cancer care.
It’s not just about removing tumors anymore. It’s about fortifying the ground they once occupied—making sure that if the enemy tries to return, it finds the terrain already claimed and fiercely defended.
In a field filled with uncertainty and relapse, this strategy offers something rare: a forward-looking defense, built not from synthetic chemicals or generic immune boosters, but from the cancer itself. It’s as personal as medicine can get—a vaccine tailored not just to a disease, but to an individual’s specific fight.
The Echoes of a Breakthrough
As the sun sets behind the red-brick laboratories of UW–Madison, Quanyin Hu and his team continue their work. They’re not chasing headlines or glory. They’re chasing something more elusive: the possibility that no patient ever has to live in fear of a cancer returning.
And in the smallest, most unlikely of places—a vesicle from a dying cancer cell—they may have found the spark that lights that future.
Reference: Zhaoting Li et al, Engineering pyroptotic vesicles as personalized cancer vaccines, Nature Nanotechnology (2025). DOI: 10.1038/s41565-025-01931-2