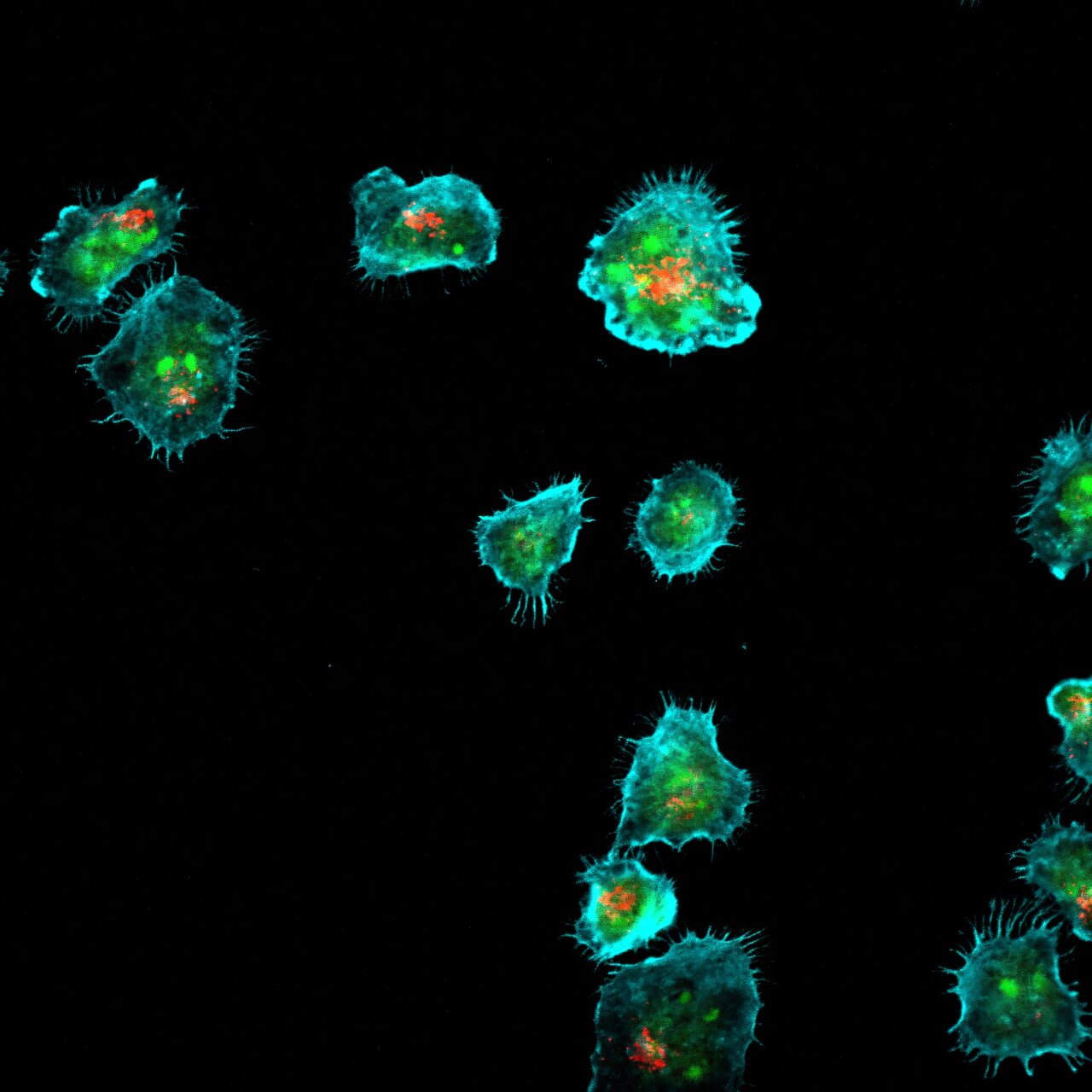
When the immune system goes to war, it does so with extraordinary precision. Each strike must be accurate—too weak, and the invader survives; too strong, and healthy tissues suffer collateral damage. At the heart of this microscopic battlefield are the body’s elite defenders: natural killer (NK) cells and T cells. These immune soldiers patrol the body, scanning for infected or cancerous cells. When they find a target, they release deadly packages—tiny toxic granules that trigger the target cell’s destruction.
Now, a groundbreaking study published in Science Immunology has uncovered an unexpected secret behind this precision attack. It turns out that lipids—fat-like molecules often associated with metabolism and energy—play a crucial role in guiding these immune assassins. The discovery not only deepens our understanding of how the immune system works but also connects immune defense with previously unrelated biological processes such as neuronal activity and lipid metabolism.
Unlocking the Hidden Machinery of Killer Cells
The research, led by Professor Kaan Boztug of the Medical University of Vienna and the St. Anna Children’s Cancer Research Institute, offers a stunning glimpse into the molecular choreography that allows NK and T cells to deliver their lethal blows. Working with an international team that included Assistant Professor Artem Kalinichenko of the Medical University of Graz and Jakob Huemer, formerly at CeMM Research Center for Molecular Medicine, the scientists used a CRISPR-based genetic screening technique to map out the genes involved in this deadly precision.
CRISPR, often described as “genetic scissors,” allows researchers to turn genes on or off with remarkable accuracy. By systematically deactivating thousands of genes, the team identified a group of previously unknown players critical to the release of cytotoxic granules. Many of these genes had something in common—they were linked to lipid metabolism.
This was an astonishing revelation. Lipids are not usually associated with the immune system’s weaponry; they are better known for building cell membranes, storing energy, and sending chemical signals. But here, lipids appeared to serve a different purpose: acting as guides and structural helpers, directing key proteins inside NK and T cells to the precise sites where toxic granules must be released.
Lipids: The Unsung Heroes of Immune Precision
To visualize what’s happening, imagine each immune cell as a skilled archer. The toxic granules are arrows tipped with deadly poison, while the lipids are the invisible hands adjusting the archer’s aim. Without them, the immune cells might miss their targets—or fail to fire at all.
The study revealed that specific lipid molecules help guide the machinery responsible for granule transport and release. When these lipids are absent or defective, the granules either fail to reach their destination or are released in the wrong direction. The result is a compromised immune response, leaving the body vulnerable to infections or cancer cells that should have been eliminated.
A Bridge Between Immunity and Metabolism
What makes this discovery particularly exciting is how it bridges two worlds once thought separate: metabolism and immunity. Lipids, long studied for their roles in energy production and cell structure, now emerge as essential components of immune defense. This insight challenges traditional scientific boundaries, showing that the same molecular pathways that shape neurons and brain function also guide the immune system’s lethal precision.
“It’s fascinating to see how molecules originally known from neuronal biology and associated with lipid metabolism and modification are also key for a distinct immune defense mechanism,” says co-first author Jakob Huemer. His observation highlights how biology reuses its tools across systems—what works in the brain may also help the immune system protect the body.
Genetic Defects and Hidden Diseases
The implications of this work extend far beyond basic biology. Many rare genetic disorders—especially those involving nerve cells and immune dysfunction—remain poorly understood. The newly identified genes could hold the missing pieces of these medical puzzles.
Patients with inherited immune deficiencies often suffer from recurring infections or struggle to fight off viruses and cancer. Similarly, some neurological diseases are linked to mutations in genes that control lipid metabolism. This study suggests that in certain cases, these conditions may stem from a shared molecular problem: a failure to deliver cellular cargo to the right place at the right time.
By mapping how lipids help control immune granule release, scientists can now better understand the molecular roots of these disorders. This could improve diagnostic accuracy for rare immune diseases and perhaps inspire new therapies that restore proper immune function.
Toward Smarter Cancer Immunotherapy
The findings also open exciting possibilities for cancer treatment. Modern immunotherapies, which harness the body’s own immune system to target tumors, rely heavily on NK and T cell function. If lipids influence how efficiently these cells kill cancerous targets, then manipulating lipid pathways might enhance immunotherapy’s effectiveness.
Professor Boztug emphasizes that this discovery goes beyond one disease or system—it changes how scientists think about biology itself. “We were able to uncover a completely unexpected connection between lipid biology and immune cell function and thereby link seemingly unrelated biological processes,” he explains. “These findings will further help us improve diagnosis of patients with rare immune defects and are also relevant for future development of cancer immunotherapy approaches.”
Curiosity-Driven Collaboration
Behind every scientific breakthrough lies collaboration and curiosity—the twin forces that drive discovery. Boztug’s team represents a network of experts from multiple institutions, united by a shared goal: to understand how life defends itself at the microscopic level. Their research combined the precision of genetic screening with detailed molecular analysis, blending technology and creativity in equal measure.
“This work showcases the power of collaborative, curiosity-driven research,” says Boztug. “By systematically exploring genetic pathways and combining functional genomics with mechanistic follow-up, we have uncovered a new group of genes that control how T and NK cells function and kill both virus-infected cells or tumor cells.”
The phrase “curiosity-driven” may sound simple, but it captures the essence of scientific progress. Often, the most transformative insights emerge not from targeted searches, but from asking questions that seem, at first, unrelated. Here, the team’s willingness to follow unexpected connections—between lipids, neurons, and immune defense—led to revelations that may reshape multiple fields of medicine.
The Hidden Elegance of the Immune System
What makes this discovery emotionally powerful is not just its medical potential, but its glimpse into the quiet elegance of biology itself. Inside each of us, millions of immune cells are constantly moving, sensing, and acting with extraordinary precision. They do not act blindly; they communicate, organize, and adapt using intricate molecular cues.
Now we know that lipids—molecules we often associate with fats or diet—are key players in this microscopic ballet. They help immune cells move with purpose, aim their attacks, and protect us from within. It’s a reminder that life’s complexity hides in the smallest details, where even a molecule of fat can determine whether a cell lives or dies.
Looking Forward
As research continues, scientists are eager to explore exactly how lipid metabolism influences immune cell behavior under different conditions—during infection, cancer, or autoimmune disease. They hope to identify ways to modulate these pathways, enhancing the immune response when needed or calming it when it turns destructive.
This study is not the end of the story but the beginning of a new chapter in immunology. It invites researchers to look at the immune system with fresh eyes—to see it not just as a set of cells and molecules, but as a living, adaptive network shaped by unexpected connections.
More information: Protein palmitoylation and sphingolipid metabolism 1 control regulated 2 exocytosis in cytotoxic lymphocytes, Science Immunology (2025). DOI: 10.1126/sciimmunol.ado3825