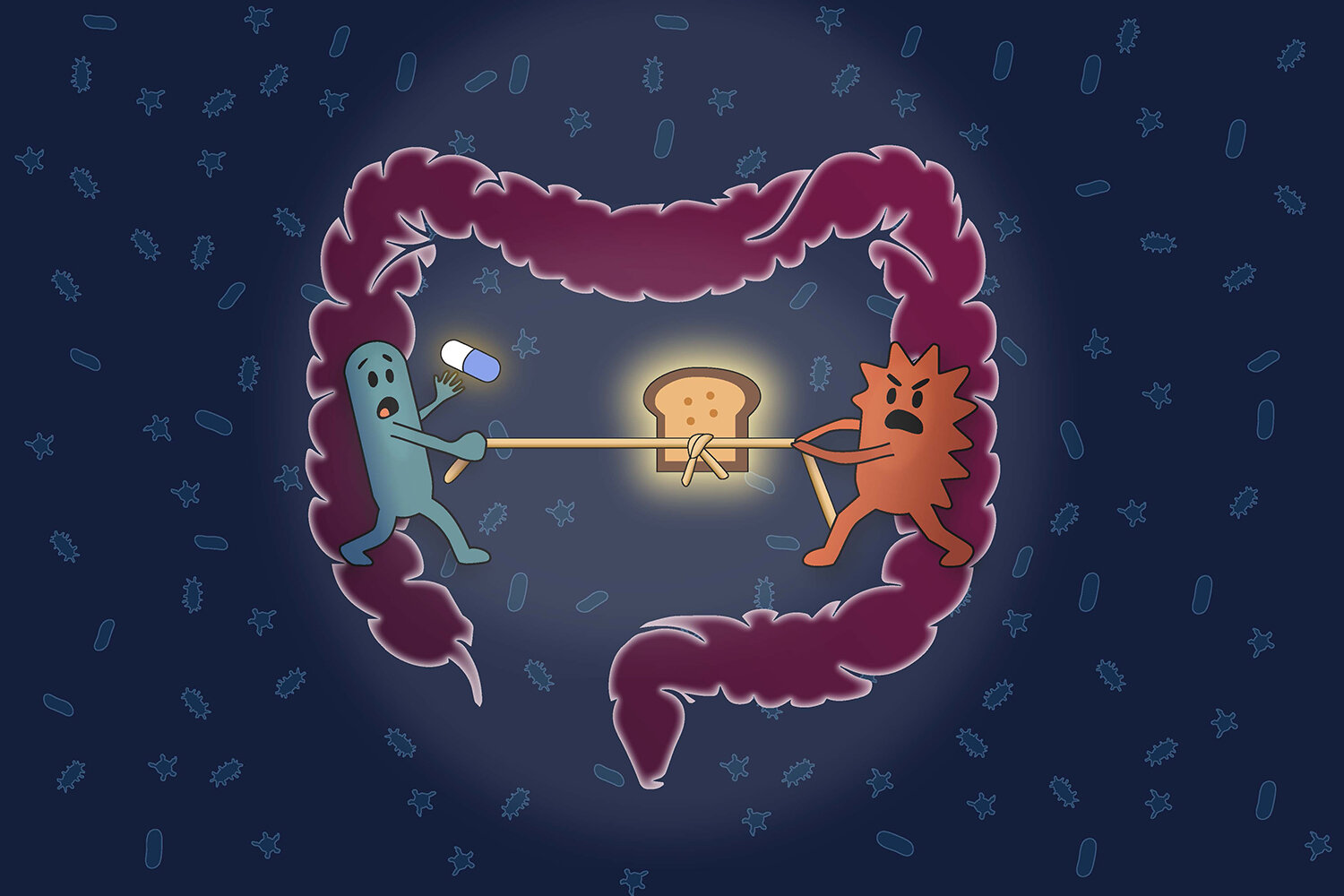
Our intestines are home to trillions of bacteria, fungi, viruses, and other microbes, all living in an intricate ecosystem that plays a critical role in keeping us healthy. These microbes help us digest food, support our immune systems, and even send chemical signals to our brain. When this microbial community is out of balance, whether due to illness, diet, or external influences, the consequences can ripple throughout our bodies, affecting everything from metabolism to mental health.
Researchers have long known that antibiotics can disrupt this delicate balance, but what about the other medications we take every day? A new study from Stanford University sheds light on this overlooked question, revealing how a wide range of common drugs—from painkillers to antidepressants—can reshape the gut microbiome, sometimes with lasting effects.
A Battle for Survival
The research, published in Cell Press on November 17, focuses on how medications alter the microbial community within the gut. The researchers set out to answer a pressing question: How do these drugs impact the trillions of microbes living in our digestive systems? To explore this, they tested the effect of 707 different clinically relevant drugs on bacterial samples taken from nine healthy human donors.
The results were striking. Among the drugs tested, 141 were found to cause significant changes in the microbiome. Some of these changes were immediate, while others persisted even after short-term drug use. In some cases, entire bacterial species were wiped out, a startling discovery that underscored the power of medications to disrupt the microbial community.
“The winners and losers among our gut bacteria can often be predicted by understanding how sensitive they are to the medications and how they compete for food,” said Handuo Shi, the postdoctoral researcher who led the study. This “fight for food,” as the researchers put it, is a central theme of their findings. Medications don’t just kill bacteria; they alter the availability of nutrients, creating a new landscape where only certain bacteria can thrive.
The study found that the primary driver of these changes was competition for nutrients. Medications, by affecting the abundance of certain chemicals in the gut, tip the balance in favor of bacteria that are better suited to take advantage of those changes. As Shi explained, “Drugs don’t just kill bacteria; they also reshuffle the ‘buffet’ in our gut, and that reshuffling shapes which bacteria win.”
Understanding the Microbial Ecosystem
The impact of drugs on our gut microbiome is complex. The researchers weren’t just interested in how individual bacteria responded to medications; they also wanted to understand how the entire ecosystem behaved. To do this, they developed data-driven computer models that predicted how bacterial communities would react to specific drugs. The models took into account the sensitivity of various species to the drug, as well as how these species competed for resources.
By using these models, the researchers were able to predict how a given drug would alter the microbiome and which bacterial species would flourish or decline. This innovative approach offers a new way to think about drug design—not just as a way to treat a disease but also as a way to preserve or enhance the health of the gut microbiome. “If we can understand and model the ecosystem response, we could one day choose drugs and accompanying diets or probiotics not only based on how well they treat a disease, but also on how they preserve or promote a healthy microbiome,” said Shi.
In this way, the study pushes us to think beyond the traditional view of drugs as isolated agents targeting individual microbes. Instead, it encourages a more holistic view, seeing drugs as tools that influence entire ecosystems of bacteria, with the potential to preserve or disrupt the balance of these communities.
Designing a Gut-Healthy Future
The implications of this research extend far beyond simply understanding how medications affect the microbiome. The goal is to use this knowledge to develop more effective, gut-friendly treatments. By understanding the complex dynamics of the microbial community, researchers hope to design medications, diets, and probiotics that can better support the health of our gut microbiome while still treating the conditions for which the drugs are prescribed.
“We’re hoping to create a future where we can design gut-healthy drug combinations that don’t just focus on the immediate effects of the treatment but also on the long-term health of the gut microbiome,” said KC Huang, the senior author of the paper. Huang, a professor of microbiology and immunology at Stanford, is passionate about the potential of this research to shape the future of medicine.
One of the next steps in this research is to explore how traditional herbal medicines, which have been used by various cultures for centuries, impact the microbiome. “Some of them are having huge effects on the microbiome,” Huang remarked. The team is testing these herbal remedies alongside conventional drugs to understand their potential benefits or risks to gut health. This could unlock a wealth of untapped resources for improving the balance of our gut microbiomes, especially in communities that rely on herbal medicines.
Furthermore, Huang envisions a future where researchers can track the microbiome in real-time as drugs are taken. By using ingestible technology to sample the small intestine, scientists could monitor changes in the microbiome as they happen, providing valuable insights into how medications alter gut health on the fly. This could be a game-changer, especially for those who experience adverse side effects from medications that affect their gut microbiome.
Why This Research Matters
Why should we care about how drugs affect the bacteria in our gut? The answer is simple: our gut microbiome plays an essential role in our overall health. Disruptions to the microbiome have been linked to a variety of conditions, including digestive issues, autoimmune diseases, and even mental health problems. Understanding how medications affect this microbial community opens up new possibilities for treating these conditions in a way that doesn’t inadvertently harm our gut health.
By rethinking how we design drugs, we can create treatments that are not only effective but also more sustainable for long-term health. This research also paves the way for personalized medicine, where treatments are tailored not just to the individual’s disease but also to their unique microbiome. As Huang put it, “It enables us to predict who is going to live, who is going to die, and makes the ensuing chaos seem really intuitive.” This ability to predict and prevent negative outcomes could be a huge leap forward in improving patient care and outcomes.
Ultimately, this study offers a new lens through which we can view the relationship between drugs and our bodies. It shows that medications are not just isolated agents—they are participants in a much larger, interconnected ecosystem. By understanding how drugs influence this ecosystem, we can take the first steps toward building a future where both medicine and microbiomes can coexist in harmony.
More information: Handuo Shi et al, Nutrient competition predicts gut microbiome restructuring under drug perturbations, Cell (2025). DOI: 10.1016/j.cell.2025.10.038