In a groundbreaking study, researchers from Texas Biomedical Research Institute and the University of Chicago have revealed a crucial mechanism that the SARS-CoV-2 virus, which causes COVID-19, uses to shield itself as it replicates and spreads within the human body. This discovery not only provides a potential avenue for developing new antiviral therapies but also offers vital insights into future vaccine design and the broader understanding of viral pathogenesis.
The study, published in Nature Communications, builds on previous research conducted by Texas Biomed, which identified key viral proteins critical to the virus’s ability to cause disease, or pathogenicity. By delving deeper into the role of these proteins, particularly one known as ORF3a, the researchers have unveiled how this protein acts as a protective shield for the virus, ensuring its continued survival and replication.
ORF3a: A Hidden Protector
In 2021, researchers from Texas Biomed first identified the accessory protein ORF3a as a critical player in SARS-CoV-2’s infectious nature. They discovered that when this protein was eliminated, the virus’s ability to cause harm to the host was significantly diminished. However, the exact mechanism behind this phenomenon remained unclear until now.
The latest study, in collaboration with the University of Chicago, has shed light on this mysterious process. ORF3a, it turns out, plays an essential role in protecting the structural proteins of the virus, most notably the spike protein, which is responsible for enabling the virus to infect other cells. The spike protein, which binds to host cell receptors and facilitates viral entry, is essential for the virus’s replication cycle. However, without proper protection, it can easily be damaged or degraded, severely impeding the virus’s ability to spread.
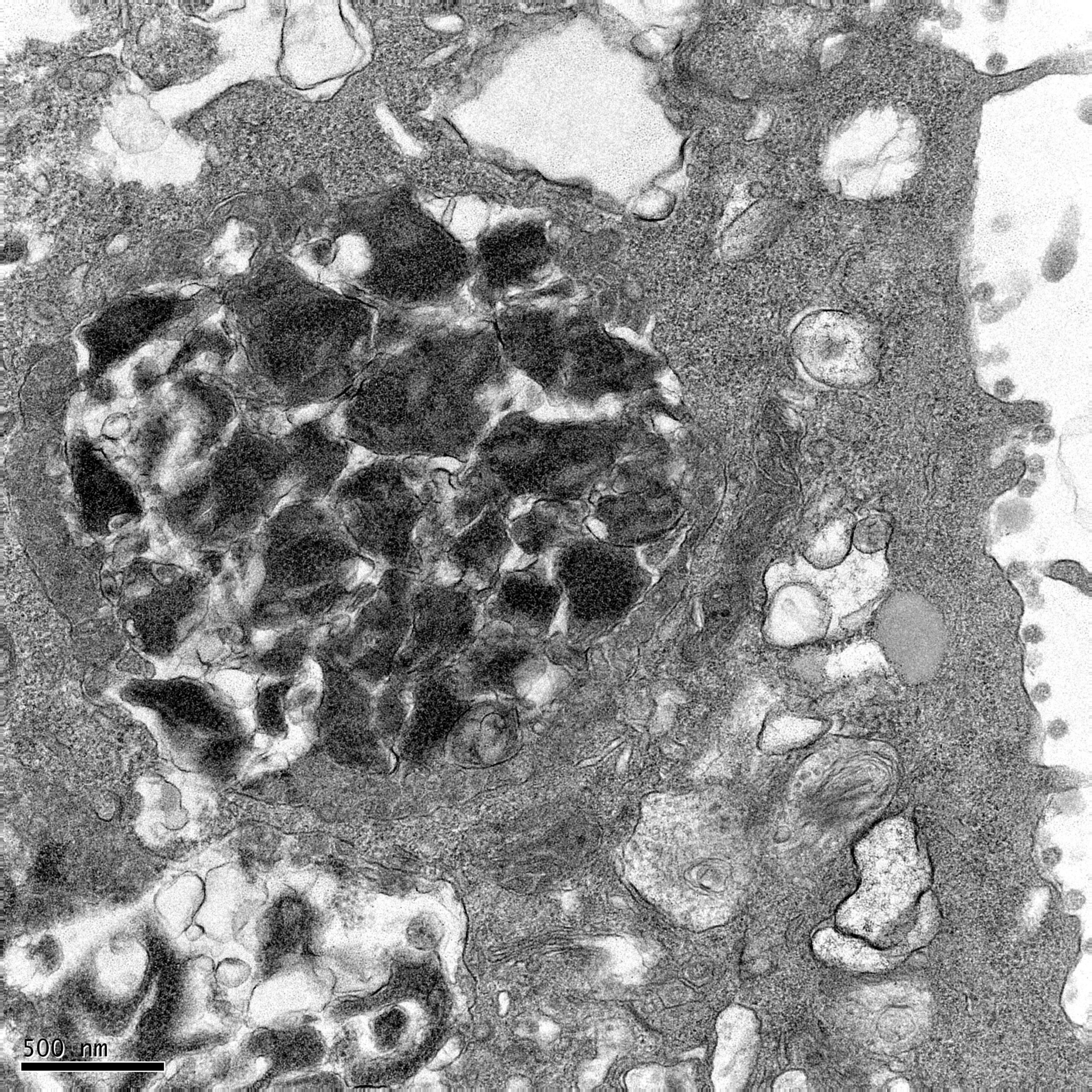
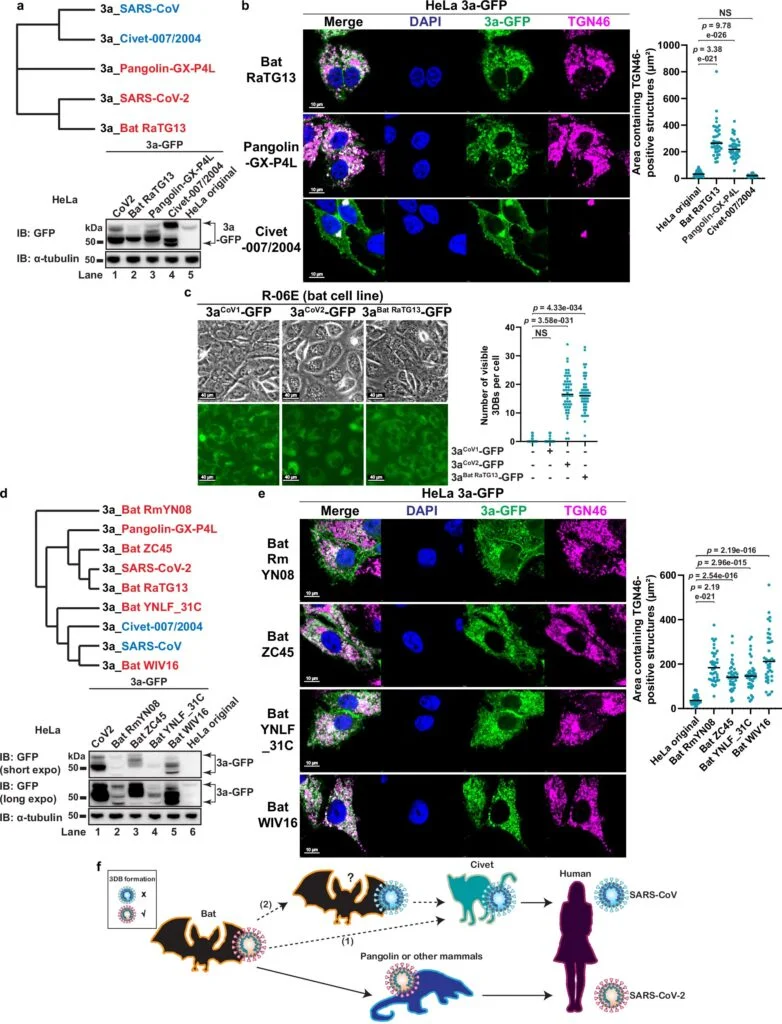
Researchers found that ORF3a accomplishes this task by triggering the formation of dense protein complexes that surround and protect the spike protein as it is assembled and transported on the surface of the viral particle. This protective mechanism works similarly to a security detail surrounding a high-profile individual or an armored vehicle, guarding the valuable “cargo”—in this case, the spike protein—during its journey to new cells. These protective complexes have been aptly named “3a dense bodies” or 3DBs by the research team.
The Role of 3DBs in Viral Success
The 3DBs formed by ORF3a are crucial for ensuring that the spike protein remains intact and functional during the replication cycle. When the ORF3a protein is absent, these protective 3DBs fail to form, leaving the spike protein vulnerable to degradation. In this scenario, the spike protein often arrives at the new cell damaged, rendering the virus less effective in its ability to infect new cells.
This discovery highlights the significance of ORF3a in the virus’s ability to protect its key structural components and ensure the success of infection. According to Dr. Luis Martinez-Sobrido, a professor at Texas Biomed, this finding provides a potential target for developing antiviral drugs aimed at blocking ORF3a’s protective function, effectively neutralizing the virus. This could also offer valuable insights into the development of vaccines that prevent the virus from assembling these critical protective complexes in the first place.
A Surprising Evolutionary Twist
While the researchers’ focus was primarily on SARS-CoV-2, their study also explored whether related coronaviruses from other species, such as those carried by bats and pangolins, also used a similar mechanism to protect their spike proteins. To their surprise, they found that these viruses do indeed form 3DBs, indicating that this protective mechanism is not unique to SARS-CoV-2. However, the researchers also discovered that other coronaviruses, such as SARS-CoV (the virus responsible for the 2003 SARS outbreak) and viruses carried by civets, do not appear to form these complexes.
This discovery adds an interesting layer to the ongoing investigation into why SARS-CoV-2 is so much more transmissible than its predecessors. The absence of 3DB formation in SARS-CoV could explain why the 2003 SARS outbreak had a much lower infection rate and a more contained transmission compared to the COVID-19 pandemic. During the 2003 SARS outbreak, only around 8,000 people were infected, while by contrast, the COVID-19 pandemic has seen more than 770 million reported cases globally, according to the World Health Organization.
The Power of Multidisciplinary Collaboration
This breakthrough is a testament to the power of interdisciplinary collaboration in science. The researchers at Texas Biomed and the University of Chicago brought together complementary expertise in virology, cellular biology, high-resolution microscopy, and virus engineering to unravel this complex process. Dr. Jueqi Chen, an Assistant Professor at the University of Chicago, led the team that identified the 3DB structures using advanced microscopy techniques, while Drs. Chengjin Ye and Luis Martinez-Sobrido at Texas Biomed provided the virology and reverse genetics expertise needed to engineer viruses and conduct functional studies.
Dr. Chen explains that their team was able to pinpoint the specific regions of the ORF3a protein that are essential for the assembly of 3DBs. This collaborative effort allowed the researchers to engineer a virus that lacked the ability to form these protective complexes, providing a deeper understanding of how the absence of ORF3a affects viral infectivity.
Future Directions: Exploring Therapeutic and Vaccine Possibilities
Now that the researchers have uncovered the role of ORF3a in protecting the virus, their next goal is to investigate what specifically causes the spike protein to break apart when it is unprotected. By identifying the factors responsible for this degradation, they hope to discover additional therapeutic targets for antiviral drugs. Furthermore, understanding how mutations in the ORF3a gene might influence the formation of 3DBs could help predict how emerging variants of SARS-CoV-2 might affect infection rates and resistance to existing treatments and vaccines.
In the coming years, the team plans to conduct further research into the behavior of different SARS-CoV-2 variants, analyzing whether mutations in the ORF3a gene alter the formation of 3DBs or affect the virus’s ability to infect cells. This could lead to the development of more effective vaccines and antiviral treatments that are tailored to specific viral strains.
A Step Toward Combating the Pandemic
This research provides a crucial piece of the puzzle in understanding how SARS-CoV-2 operates at a molecular level. By uncovering the protective mechanism that allows the virus to thrive and spread within the body, researchers have identified a potential target for drug development and vaccine strategies that could disrupt this process.
The study also underscores the importance of continued research in the fight against COVID-19. With millions of cases and countless lives impacted globally, it’s more important than ever to uncover new ways to neutralize the virus and prevent its transmission. The discovery of ORF3a’s role in protecting the spike protein marks a significant milestone in this ongoing battle, offering hope that the lessons learned from this research could lead to more effective treatments and vaccines in the future.
As the scientific community continues to grapple with the complexities of COVID-19, discoveries like this one shine a light on the potential pathways for combating the virus and its ever-evolving variants. The fight is far from over, but with each new finding, we move one step closer to overcoming the pandemic.
Reference: Stella Hartmann et al, SARS-CoV-2 ORF3a drives dynamic dense body formation for optimal viral infectivity, Nature Communications (2025). DOI: 10.1038/s41467-025-59475-x