Lung cancer remains one of the most devastating diseases in the world—a silent intruder that often grows unnoticed until it’s too late. For decades, scientists have been trying to understand how it begins, how normal lung cells turn rogue, and how to stop that transformation before it spirals out of control.
Now, researchers at The University of Texas MD Anderson Cancer Center have taken a remarkable step toward answering that question. By creating some of the most detailed molecular maps ever made of lung cancer development, they have discovered something profoundly revealing: inflammation may be one of the earliest and most powerful drivers of lung cancer’s birth.
Their findings, recently published in Cancer Cell, not only reshape our understanding of how lung cancer starts but also point toward new strategies to intercept it—long before it becomes deadly.
The Power of Seeing at the Cellular Level
Every disease tells a story, and cancer’s story is written deep within our cells. But until recently, scientists could only read fragments of that story. Traditional techniques allowed researchers to see which genes were active in a tumor but not where they were active within the complex architecture of tissue.
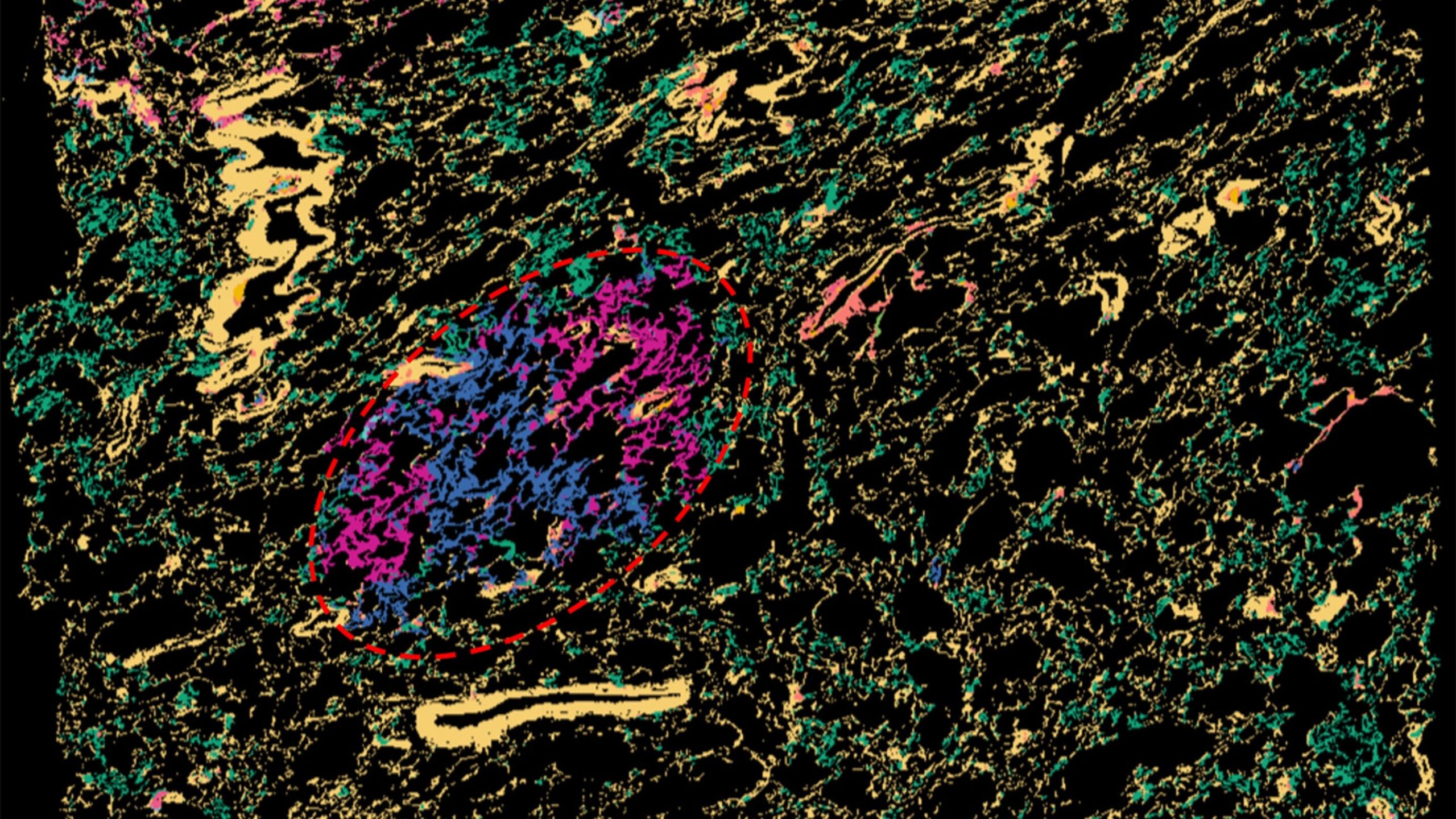
That limitation has changed with a revolutionary technology known as spatial transcriptomics. This method lets scientists map how genes are expressed—cell by cell, region by region—within a tissue sample. It’s like moving from a blurry, black-and-white sketch to a vivid, high-definition photograph.
Led by Dr. Humam Kadara, professor of Translational Molecular Pathology, and Dr. Linghua Wang, professor of Genomic Medicine and associate member of the James P. Allison Institute, the MD Anderson team used this technology to map the genetic and cellular landscape of lung tissue before and during the development of cancer. Their goal was simple yet profound: to see the invisible, to watch the first molecular sparks of cancer flicker into life.
Uncovering a Surprising Culprit: Inflammation
What the researchers found could change how we think about cancer prevention. The earliest signs of lung cancer, they discovered, were surrounded by zones of intense inflammation—regions where immune cells were unusually active and proinflammatory signals were abundant.
“We find that the earliest cells that give rise to lung cancer are in regions with very high inflammation and are surrounded by proinflammatory cells,” said Dr. Kadara. “Targeting inflammation by neutralizing a driver called IL-1B reduces these precursor cells of lung cancer.”
IL-1B, short for interleukin-1 beta, is a molecule known for its role in the body’s immune response. It helps trigger inflammation to fight off infections or repair tissue damage. But when this defense mechanism becomes overactive or prolonged, it can do harm—fueling genetic instability, damaging DNA, and creating an environment where cancer cells can emerge and thrive.
The team’s findings suggest that inflammation isn’t just a byproduct of cancer—it may be one of its earliest triggers.
Creating a Map of Early Cancer
To visualize this process in unprecedented detail, the researchers built spatial transcriptomic maps of 56 human lung samples, including both precancerous lesions—tiny, early tissue abnormalities—and more advanced cancerous tissues from 25 patients. Each map was a microscopic city of cells, with every gene expression pattern serving as a coordinate in a living atlas.
Then, to make sure their results were accurate, the team validated their discoveries using an independent group of 36 additional samples from 19 patients. In total, the analysis encompassed over 486,000 regions and 5.4 million individual cells.
What emerged from these maps was a vivid picture of early lung cancer evolution. The precancerous regions weren’t just clusters of abnormal cells—they were ecosystems, teeming with interactions between lung cells and immune cells, bathed in a persistent inflammatory environment.
Inflammation as the Spark of Transformation
Within these regions, certain lung cells known as alveolar cells—the delicate structures that exchange oxygen and carbon dioxide—appeared to be particularly vulnerable. When surrounded by inflammation, these cells began showing the earliest genetic signs of transformation into cancerous cells.
The study found that inflammatory hotspots were more active and prevalent in the earliest stages of lung cancer development and were conserved even in laboratory models. This pattern strongly suggests that inflammation acts as a spark, initiating cellular changes long before a detectable tumor forms.
This insight may explain why people with chronic lung inflammation—due to smoking, air pollution, or conditions like chronic obstructive pulmonary disease (COPD)—are at higher risk of developing lung cancer. It’s not just that their lungs are damaged; it’s that the persistent inflammatory state may be setting the stage for malignant change.
Turning Discovery into Hope
Understanding that inflammation drives early cancer development opens a new frontier for prevention. If inflammation can be controlled or neutralized, perhaps lung cancer can be stopped before it even begins.
The MD Anderson researchers found that neutralizing IL-1B, one of the key inflammatory molecules in these early lesions, reduced the number of lung cancer precursor cells. This is an extraordinary finding—it suggests that targeting inflammation itself could serve as an interception strategy, halting cancer before it takes root.
This idea aligns with growing evidence from other studies showing that anti-inflammatory therapies may reduce cancer risk. In fact, some clinical trials with drugs that block IL-1B have already shown decreased cancer incidence as a side effect, hinting that this approach could one day become part of mainstream cancer prevention.
A New Vision for Cancer Prevention
Traditionally, medicine has focused on detecting and treating cancer once it becomes visible—through scans, biopsies, or symptoms. But by that point, cancer has already had years to grow and evolve. The new vision emerging from studies like this one is interception: identifying and stopping cancer at its molecular inception, before it ever turns deadly.
Spatial transcriptomics makes this vision possible. By revealing where and how dangerous changes occur in tissues, it provides a roadmap for scientists to design therapies that intervene at just the right time. Instead of waiting for cancer to appear, doctors could one day use these maps to identify high-risk patients and deliver targeted anti-inflammatory treatments before tumors form.
From the Lab to the Patient
While this study represents a groundbreaking scientific advance, its true significance lies in its potential impact on patients. Every insight gained brings the world closer to personalized prevention—the ability to predict who is at risk, why, and how to stop it.
For lung cancer, a disease often detected too late, this could be life-changing. Early intervention strategies that focus on reducing inflammation might drastically lower the number of cases that ever reach the stage of invasive cancer. Combined with advances in imaging, genetic testing, and artificial intelligence, this approach could revolutionize how we fight the disease.
The Bigger Picture: Inflammation and Cancer Everywhere
Although this research focuses on lung cancer, its implications reach far beyond the lungs. Chronic inflammation has long been linked to many other cancers, including those of the liver, colon, and stomach. Understanding the exact molecular dialogue between inflammation and cancer cells could open the door to broad-spectrum preventive therapies—treatments that cut off cancer at its inflammatory roots across multiple organs.
This represents a profound shift in how we think about cancer prevention. Instead of chasing cancer after it appears, we may be able to treat the soil instead of the seed—to calm the inflammatory storm that gives cancer its first opportunity to grow.
More information: Fuduan Peng et al, Multimodal spatial-omics reveal co-evolution of alveolar progenitors and proinflammatory niches in progression of lung precursor lesions, Cancer Cell (2025). DOI: 10.1016/j.ccell.2025.10.004