On a quiet morning at the DGIST campus, a team of scientists peered through microscopes and computer simulations, chasing a dream that felt almost like alchemy: turning sunlight and carbon dioxide into something valuable, something that could change the way humanity powers itself. Carbon dioxide, the silent culprit behind climate change, had long seemed unstoppable. But now, it faced an opponent that did not roar or hum, but shimmered under the light, bending photons into energy for change.
The idea was deceptively simple. Sunlight carries immense energy, enough to drive life itself. If only we could capture it efficiently and direct it, perhaps we could transform carbon dioxide, a greenhouse gas, into methane, a high-value fuel. But simple ideas rarely survive contact with reality. For decades, attempts at artificial photosynthesis had stumbled over limitations. Materials remained stubbornly crystalline, electrons got trapped, and active sites—the places where chemical magic occurs—were too few. Efficiency lagged, and hope dimmed.
A Spark of Inspiration Across Continents
In a collaboration that bridged continents, Professor Suil In from DGIST joined forces with Professor William A. Goddard III of Caltech. Together, they pursued a dream that spanned oceans and laboratories. Their goal was to replicate nature’s brilliance, to mimic the subtle elegance of photosynthesis itself.
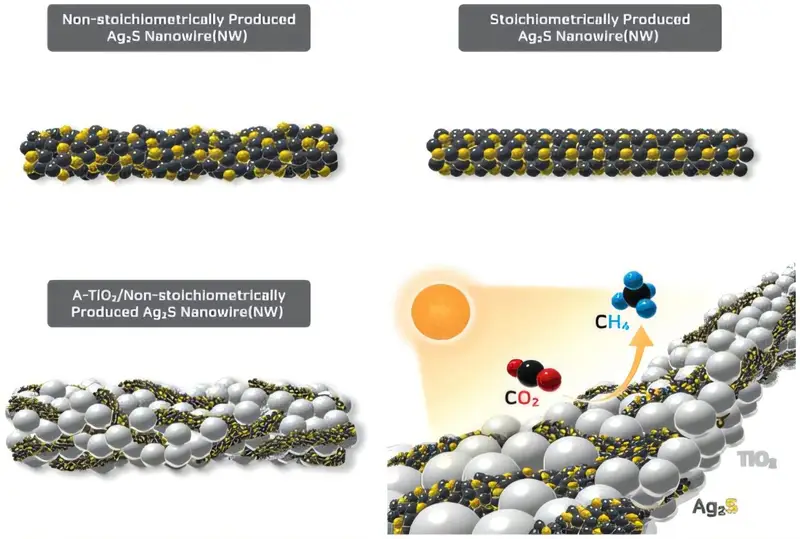
“We wanted to understand how sunlight could not just touch matter but move through it, channeling energy in the most effective way,” Professor In explained. Their approach combined silver sulfide, a material that soaks in visible and near-infrared light, with titanium dioxide, a staple in photocatalysis. But rather than layering them together conventionally, the team designed a new structure that allowed electrons to travel efficiently through a Z-scheme—a pathway inspired by the very process plants use to convert light into chemical energy.
The result was more than an incremental improvement. Light, which once scattered uselessly, now had a highway. Electrons surged, energy flowed, and the potential for chemical transformation surged along with it.
Embracing Imperfection to Unlock Power
Yet the breakthrough did not stop at efficiency. The team made a counterintuitive choice: they introduced defects deliberately. Where others sought perfect crystals, these scientists sought irregularity. Amorphous titanium dioxide formed irregular structures brimming with titanium tertiary active sites, places where carbon dioxide could cling and react. Non-stoichiometric silver sulfide nanowires, whose atomic ratios were intentionally mismatched, created powerful internal electric fields.
“Defects are not simply structural limitations,” the researchers discovered. “They can actually be a key factor in improving catalyst performance.”
This embrace of imperfection turned the photocatalyst into a microscopic workshop, where sunlight, electrons, and carbon dioxide danced in harmony. Charge separation improved dramatically, reaction sites multiplied, and the previously sluggish process surged forward with unprecedented speed.
Methane Emerges From the Light
The proof was in the numbers. In a concentrating reactor environment, the newly developed photocatalyst achieved a methane production rate of 30.31 μmol/g—roughly five times greater than ordinary conditions. The transformation was no longer theoretical. Sunlight, carbon dioxide, and carefully engineered materials had converged to produce fuel, a tangible testament to human ingenuity.
Professor In reflected on the achievement, “This research is significant as it suggests the possibility of designing and controlling the ‘active sites’ that determine catalyst efficiency. This will contribute to quickening the commercialization of the technology that converts carbon dioxide into valuable fuel.”
What made this success even more remarkable was the clarity with which the team understood the process. Using a combination of experimental observation and quantum mechanical calculations, they traced the path of electrons and molecules at the atomic level, revealing precisely how carbon dioxide transforms into methane. This was science not just as invention, but as revelation—a peek behind the curtain at nature’s most intimate mechanics.
Why This Discovery Matters
In the fight against climate change, the stakes could not be higher. Carbon dioxide levels continue to rise, and humanity’s reliance on fossil fuels threatens both ecosystems and economies. Yet here, in a lab where sunlight, silver, and titanium meet, a solution glimmers. This photocatalyst represents more than an elegant experiment. It embodies the possibility of turning waste into energy, of capturing sunlight to fuel our world without producing further emissions.
The implications are profound. With continued development, technologies like this could accelerate the shift to carbon-neutral energy, transforming solar light into a resource capable of sustaining both industry and life. The research demonstrates that innovation can emerge from embracing imperfection, that understanding at the atomic level can yield tools of global significance, and that collaboration across borders can turn dreams into reality.
This work is a reminder that science, at its best, is a story of hope. It shows that the challenges of climate change, daunting as they are, can be met with creativity, precision, and relentless curiosity. Sunlight, once merely the backdrop of life, can become the sculptor of a sustainable future. Carbon dioxide, once a symbol of human excess, can be turned into a fuel that powers progress.
And in that transformation, both nature and humanity are reflected, working together in a dance that is as beautiful as it is necessary.
More information: Niket S. Powar et al, Defect-Driven Dynamics in Gas-Phase Photocatalytic CO2 Conversion to Solar Fuels Using Ti3+/Ti4+Containing TiO2and Nonstoichiometric Ag2S Nanowires, ACS Catalysis (2025). DOI: 10.1021/acscatal.5c05258