Pain is supposed to end. Wounds are meant to close. The body, resilient and wise, has evolved countless mechanisms to survive injury, to repair, to move forward. But sometimes healing isn’t the end. Sometimes, long after the bruises fade and the stitches are removed, something deeper lingers—something the eyes can’t see, but the body never forgets.
A groundbreaking new study from researchers at the University of Toronto Mississauga, published in Current Biology, reveals that past physical injuries can leave a lasting imprint—not only on the body but on the mind and nervous system. This residual echo of trauma can prime an individual to overreact to future stressors, triggering exaggerated fear and chronic pain, even in the absence of new injury.
The research, led by Dr. Loren Martin and graduate student Jennet Baumbach, challenges the traditional view of pain as a transient response to damage. It paints a new, more haunting picture: that injury can teach the nervous system to be afraid—of everything. And that fear can set in motion a cascade of biological changes that keep the body locked in a state of heightened sensitivity for months, even years.
A Fractured Response to Fear
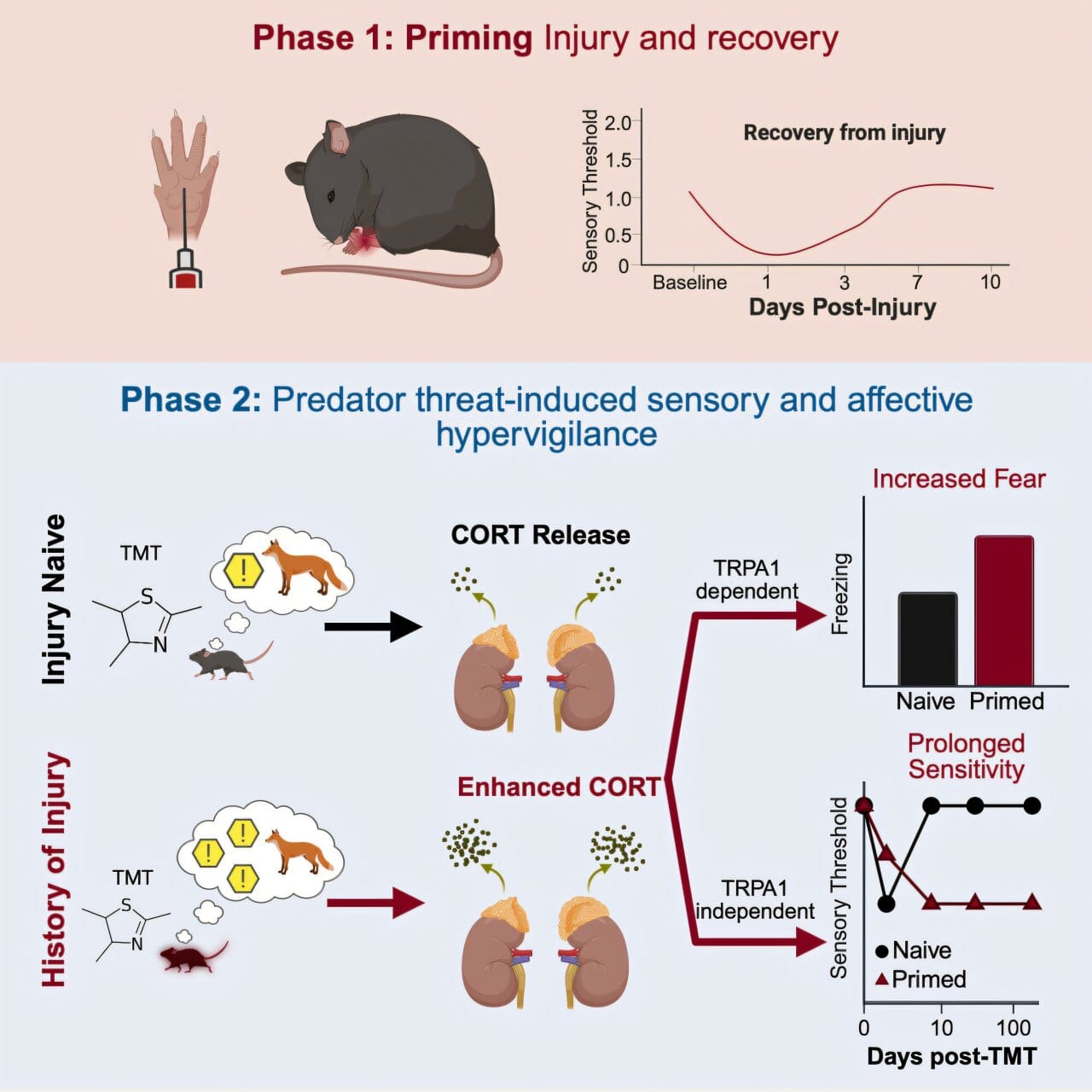
The study’s revelations began with a simple but powerful observation in mice. After a routine injury to one paw, researchers allowed the animals to recover fully. But when the mice were later exposed to the scent of a predator—a terrifying stimulus in the animal world—their bodies responded not just with fear, but with pain. Both hind paws became hypersensitive, including the uninjured side.
More remarkably, the pain didn’t vanish once the predator odor disappeared. It lingered, chronic and mysterious, for over six months—a significant portion of a mouse’s life. The physical wound had long since healed. But something had changed in the wiring of the brain.
“The response was not just about memory,” said Dr. Martin. “It was a physical, behavioral, and neurochemical transformation. The mice weren’t just remembering danger—they were reliving it in their bodies.”
This mirrored the experiences of countless people living with chronic pain. Pain that doesn’t make sense. Pain without injury. Pain that flares with stress, exhaustion, or fear. For decades, such cases were often dismissed or misunderstood, attributed to anxiety or even malingering. Now, thanks to studies like this one, science is beginning to understand the truth: the nervous system can be trained to suffer.
Fear and Pain: Two Paths, One Road
To unravel the biological threads binding stress and pain, the research team zeroed in on two key players: the stress hormone corticosterone (a cousin of cortisol in humans) and a protein known as TRPA1, sometimes called the “wasabi receptor” for its ability to generate a sharp, burning sensation in response to irritants.
When the mice smelled the predator, corticosterone levels surged. In animals with a history of injury, this hormonal flood activated TRPA1 in key pain-sensing neurons, essentially lighting up the brain’s alarm system. It was as if the body screamed: “Danger is here again—prepare to hurt!”
Jennet Baumbach, the study’s first author, noted the elegance of the mechanism. “TRPA1 and corticosterone appear to form a loop, reinforcing the message that the world is dangerous. The more stress, the more activation. The more activation, the more fear and pain.”
But there was an unexpected twist. While both corticosterone and TRPA1 were necessary for the intense fear response, the prolonged pain that followed depended solely on stress hormones. TRPA1 wasn’t involved in the lingering discomfort. This means fear and pain are not simply two expressions of the same process; they are siblings traveling the same road in parallel, shaped by shared trauma but governed by distinct biology.
The Biology of a Haunted Nervous System
What this study lays bare is that injury is not just about damaged tissue—it’s a lesson the body learns. Pain, at its core, is a survival tool. When we’re hurt, our brain stores the event like a warning sign on the road map of life: “Avoid this. Remember this.” But sometimes, the brain overlearns. It rewires itself too well. The protective signal becomes pathological.
Stress hormones like corticosterone amplify the response, flooding the body with molecules that increase sensitivity in neurons, modulate immune cells, and ramp up vigilance. These effects are useful in short bursts—but toxic when constant. Chronic stress becomes a fuel source for chronic pain, anxiety, and hypersensitivity.
TRPA1, for its part, may serve as a sensory gatekeeper, enhancing the perception of pain during acute danger. It’s expressed in sensory neurons and reacts to environmental irritants like smoke, cold, or pungent chemicals—anything that might harm us. In the context of prior injury, it appears to “remember” that the body was once hurt, keeping the alarm bells just beneath the surface.
But the real revelation is this: you don’t need to be hurt again to feel hurt again. All it takes is the memory of threat—or a trigger that mimics it—to reawaken the pain.
A Window Into Human Suffering
Though conducted in mice, the implications of this research for human health are profound. It offers a window into the biology of conditions like fibromyalgia, complex regional pain syndrome, and post-traumatic stress disorder, all of which are characterized by exaggerated pain or emotional responses following trauma.
For people with chronic pain, the findings offer something beyond science—they offer validation. Many patients report that stress, even without physical exertion, makes their pain worse. Some say loud noises, strong smells, or arguments can trigger flares. These aren’t coincidences or quirks. They are echoes of an altered nervous system.
“Understanding how trauma rewires the brain is crucial,” Dr. Martin said. “We can’t fix what we don’t understand. But if we know the mechanisms, we can begin to unravel them.”
Already, the research suggests possible new treatments. Blocking corticosterone signaling, or inhibiting TRPA1, may reduce hypersensitivity and help retrain the nervous system to respond more appropriately. Future drugs might target these molecules directly, or indirectly via stress reduction, neuroplasticity, or immune modulation.
The Quiet Revolution of Neuroscience
This study is part of a broader revolution in neuroscience—a shift from seeing the brain as a static organ to understanding it as a dynamic, plastic system constantly shaped by experience. Trauma, injury, fear, and stress don’t just influence the mind; they sculpt the body. They leave fingerprints in the architecture of the nervous system.
And just as these patterns can be etched in, researchers believe they can be eased out. The brain’s ability to change—neuroplasticity—is both the problem and the solution. While trauma can wire us for fear and pain, healing may rewire us for resilience.
The future of pain management, then, lies not only in numbing symptoms but in decoding the stories the body tells itself. This study is a step in that direction—a way to make sense of suffering that often defies logic.
Where Science Meets Compassion
Perhaps the most powerful message of this research is one of empathy. It reminds us that the human body is a storyteller. It remembers slights, injuries, and terrors long after they’ve passed. It encodes them not just in scars or memories, but in reflexes, hormones, and neurons. And when it reacts, it does so not out of weakness, but out of a deep desire to survive.
Understanding this doesn’t just change medicine—it changes how we treat each other. It calls for a gentler kind of science. One that listens. One that believes. One that recognizes that healing isn’t always linear—and that sometimes, the greatest wounds are the ones we can’t see.
In a world that rushes to fix what it can’t feel, studies like this offer a quiet revolution. They teach us that what hurts us can shape us—but it doesn’t have to define us.
And with that understanding comes the first glimmer of relief.
Reference: Jennet L. Baumbach et al, A history of injury enhances affective and sensory responses to predator threat by sensitizing corticosterone release through TRPA1 receptor signaling, Current Biology (2025). DOI: 10.1016/j.cub.2025.07.005