Long before we speak our first words, feel our first heartbreak, or lose ourselves in joy or despair—long before we even open our eyes—the architecture of our brain is already drafting the story of who we may become. Inside the growing womb, in the silent corridors of the developing fetal brain, stem cells divide, communicate, and choose their destinies. It is here, at this embryonic frontier, that the seeds of complex mental and neurological disorders may be planted—seeds that will not bloom for decades, or perhaps, not at all without the right trigger.
This revelation doesn’t just expand the horizon of medical science. It rewrites it.
A groundbreaking study led by the Hospital del Mar Research Institute in Barcelona and Yale University has brought to light compelling evidence that the origins of disorders like autism, depression, schizophrenia, bipolar disorder, and even Alzheimer’s and Parkinson’s may begin far earlier in life than we ever imagined—perhaps even before life feels like life at all. Published in Nature Communications, this work doesn’t just follow a new thread in neuroscience; it charts a whole new tapestry.
A Question that Reaches Back in Time
The research was driven by a deceptively simple question: when do the biological roots of mental illness truly begin?
Most studies of neuropsychiatric and neurodegenerative disorders focus on adult brains, the logic being that we should examine disease at its peak. But Dr. Gabriel Santpere, the study’s lead researcher and head of the Neurogenomics Research Group at the Hospital del Mar Research Institute, believed this view was too narrow. The brain, after all, doesn’t spring into being at adulthood. It is forged across an elaborate developmental timeline, from the womb onward, guided and shaped by a symphony of genetic instructions.
Santpere and his colleagues proposed a bold hypothesis—that many of the genes associated with brain disorders don’t wait until adolescence or adulthood to influence our fate. Instead, they begin to express themselves in the earliest stages of brain development, specifically in neural stem cells—those tireless builders that give rise to every neuron and support structure in the brain.
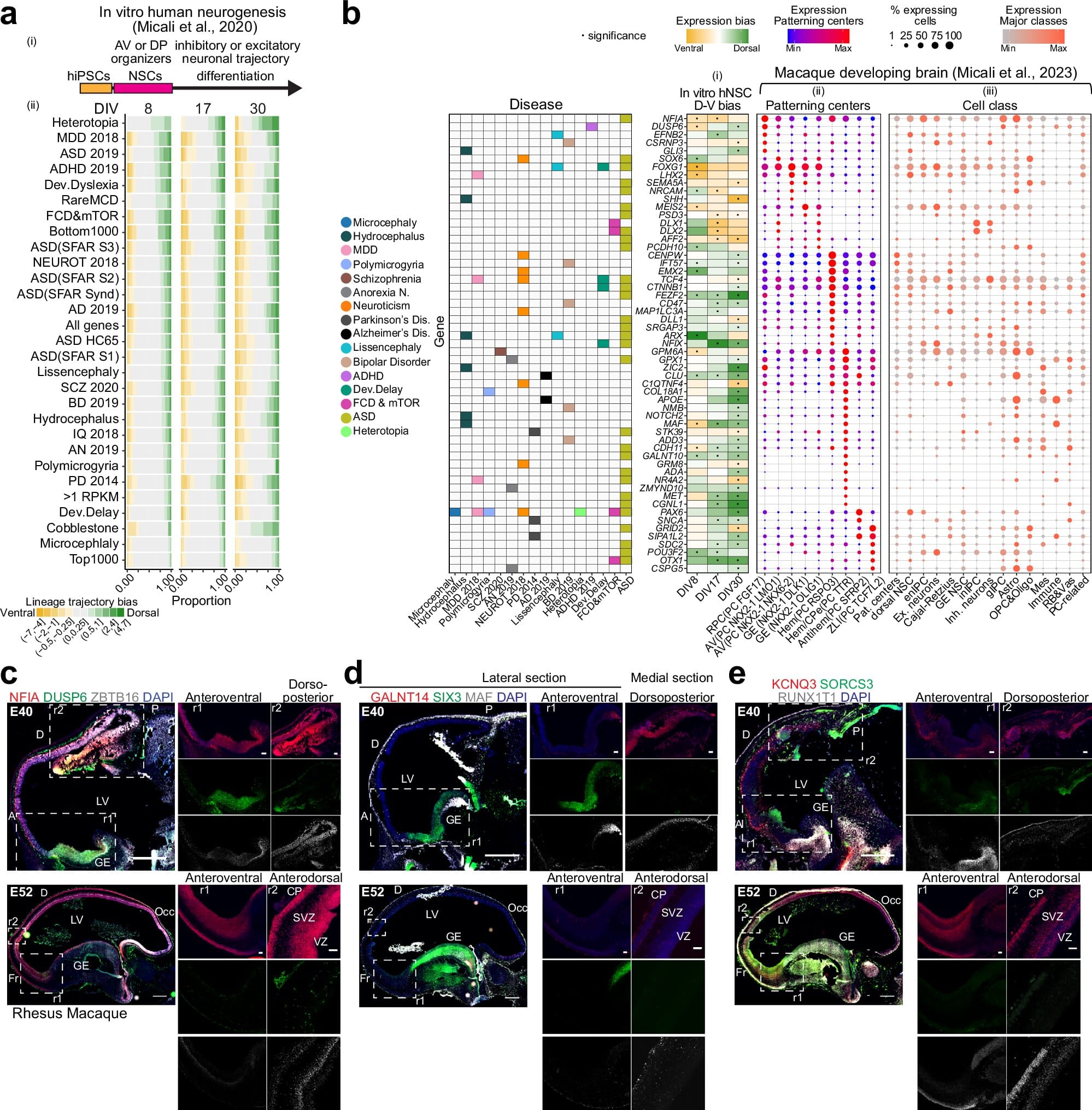
To test this, they turned to the deepest levels of biology, using high-resolution data from both human and mouse brains, and cellular models grown in the lab. By simulating how nearly 3,000 genes associated with neuropsychiatric and neurodegenerative conditions behave in fetal brain cells, they looked back in time, to the beginning of everything.
What they found was striking. A great number of these genes were already active in the fetal brain, influencing the decisions and fate of neural stem cells at critical moments. And when these genes were altered—either turned on too early, too late, or not at all—the entire blueprint of the brain could change.
Brains in the Making, Futures in the Balance
Understanding how mental illness takes root in the brain is like trying to catch a shadow on the move. Most conditions like schizophrenia or Alzheimer’s don’t emerge until later in life, yet their underlying causes may be hardwired into the brain decades earlier. The power of this study lies in its ability to time-travel—to reach into the fetus’s developing brain and ask: what’s happening here that could shape the future?
The researchers built intricate simulations of the brain’s earliest regulatory networks—systems that dictate how and when certain genes are turned on within specific types of cells. They did this not in isolation, but by layering developmental timelines, brain regions, and cell types, creating a multidimensional map of how these thousands of genes might influence the brain’s assembly.
This approach revealed a stunning insight: many of the genes implicated in autism, depression, anorexia, bipolar disorder, and schizophrenia are already active in neural stem cells during fetal development. This isn’t to say these genes immediately cause disease—but that their timing, coordination, and cellular targets are crucial. One small misstep in this tightly choreographed genetic ballet could ripple outward, shaping brain circuits that are subtly miswired, leaving a person more vulnerable to mental health struggles later in life.
A Fragile Dance of Genes and Time
Co-lead researcher Dr. Nicola Micali from Yale University described the challenge poignantly. “Scientists usually study the genes of mental illnesses in adults,” he explained, “but in this work we discovered that many of these genes already act during the early stages of fetal brain formation.”
Their findings shine a new light on the brain’s formative period. The fetal brain is not a passive canvas waiting to be painted, but an active, bustling workshop where every minute counts. It is during this time that stem cells differentiate into neurons, travel to the correct brain regions, and form the circuits that will eventually control thought, emotion, memory, and behavior.
And like any construction project, timing is everything.
If a gene that helps stem cells become inhibitory neurons activates too late, the delicate balance of brain excitability might be lost—potentially contributing to disorders like epilepsy or schizophrenia. If a gene that supports synapse formation is overexpressed too early, it could disrupt the network wiring that underpins social cognition—possibly leading to features of autism.
By identifying the exact moments and cell types where these genes exert their greatest influence, the study offers something precious: a map. Not just of where things go wrong, but of when. These temporal windows are now targets—opportunities for future therapies that could intervene early, perhaps even before symptoms arise.
Disorders of the Future, Rooted in the Past
The researchers didn’t limit themselves to psychiatric conditions. They also explored genes associated with cortical malformations like microcephaly and hydrocephaly, and with age-related neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Astonishingly, these too showed signs of involvement in the earliest phases of brain development.
This challenges the long-standing belief that such disorders are primarily diseases of old age. If genes related to Alzheimer’s begin influencing brain development in utero, then the disease may be a lifelong process, not simply a late-life deterioration. It means that Alzheimer’s prevention may need to start far earlier than previously imagined—perhaps even before birth.
Dr. Xoel Mato-Blanco, a researcher on the project, emphasized this point, saying, “We cover a wide spectrum of diseases that the brain can have and look at how the genes involved in these conditions behave in neural stem cells.”
The implications are enormous. They call on society to rethink how we approach brain health—not as something to treat reactively in adulthood, but as something to safeguard from the very beginning of life.
Toward a New Frontier in Medicine
This research doesn’t just raise the curtain on a hidden act of brain development; it opens a new frontier for clinical medicine. By pinpointing which genes act when—and in which cell types—we can begin to design targeted therapies that are more precise, more personalized, and more preventative.
In the age of gene editing and regenerative medicine, the idea of intervening during or shortly after fetal development no longer belongs solely to the realm of science fiction. With enough understanding, it may one day be possible to correct or modulate the expression of at-risk genes before they lead to disease.
Dr. Santpere envisions this future: “Understanding how genetic alterations translate into pathology helps develop targeted therapies. It opens the door to gene therapy and personalized medicine tailored to the individual’s developmental profile.”
These aren’t just dreams. They are blueprints waiting to be realized—much like the developing brain itself.
The Soul in the Cells
Behind every scientific paper lies a deeper truth—one that reaches beyond molecules and mechanisms. This study reminds us that mental illness is not a moral failing, not a flaw of character or weakness of will. It is biology, shaped by genes and time, environment and fate. It is written into the fabric of who we are before we ever have a name.
To see that—to trace the roots of suffering back to a moment before thought, before self-awareness—is both humbling and hopeful. It means we may be able to change the trajectory of illness before it takes hold. It means we can see people not as broken, but as beautifully complex beings whose stories began long before they could ever tell them.
In the quiet workshop of the fetal brain, stem cells labor in silence. Now, thanks to science, we are beginning to understand what they were trying to say all along.
Reference: Xoel Mato-Blanco et al, Early developmental origins of cortical disorders modeled in human neural stem cells, Nature Communications (2025). DOI: 10.1038/s41467-025-61316-w