When we think of Parkinson’s disease, we often picture the visible signs—shaking hands, slowed movements, and stiffness. But behind these physical symptoms lies an invisible battle unfolding within the brain’s electrical rhythms. Understanding this battle has challenged scientists for decades. Now, a groundbreaking collaboration between leading European research centers has taken a major step toward decoding how the brains of Parkinson’s patients generate these symptoms—and how that knowledge could transform treatment.
A Silent Symphony of the Brain
Our brains are alive with electrical activity. Billions of neurons communicate through tiny pulses, creating oscillations—waves that rise and fall at different frequencies, like the synchronized instruments in a symphony. In Parkinson’s disease, something disrupts this harmony. Instead of smooth coordination, the neural orchestra falters, producing disordered rhythms that lead to tremors, rigidity, and difficulty initiating movement.
One of the main suspects in this neurological chaos is the beta wave—a type of brain oscillation that pulses about 20 times per second. For years, researchers have suspected that the strength of these beta waves reflects the severity of a patient’s motor symptoms. Stronger beta activity seems to go hand-in-hand with slower, stiffer movements. But evidence has been inconsistent. Some studies showed a clear relationship; others did not. The reason for these discrepancies has long puzzled scientists.
The Power of Collaboration
A team led by the Max Planck Institute for Human Cognitive and Brain Sciences in Leipzig decided to find out why. Partnering with some of Europe’s most advanced clinical centers—including Charité Berlin, Heinrich-Heine University Düsseldorf, University College London, and the University of Oxford—they undertook one of the most comprehensive studies ever conducted on Parkinson’s brain activity.
The collaboration focused on patients already receiving deep brain stimulation (DBS), a therapy that uses implanted electrodes to deliver tiny electrical impulses to specific brain regions. DBS can dramatically reduce symptoms for people with severe Parkinson’s disease. But these same electrodes also provide a rare scientific opportunity: they can record electrical activity directly from deep brain areas that would otherwise be inaccessible in living humans.
The researchers realized that by combining these recordings from multiple hospitals, they could create a far larger and more powerful data set than any single study had achieved before. They standardized their analysis methods and synchronized their equipment, allowing them to compare results across more than 100 patients—a first in the field.
The Hidden Role of Sample Size
What they found was both enlightening and humbling. The link between beta wave activity and symptom severity was real—but much weaker than previously believed. Earlier studies had reported conflicting results not because they used the wrong equipment or flawed analysis methods, but because they didn’t include enough participants to detect such subtle effects.
In the intricate landscape of the brain, even strong patterns can appear faint when viewed in small samples. Only by pooling data from many patients could the researchers reveal a clear, statistically reliable connection between beta activity and Parkinson’s movement symptoms. This discovery highlights a crucial principle of neuroscience: when studying the brain’s complex dynamics, size truly matters.
Listening Beyond the Noise
But there was another reason for past inconsistencies—one that had less to do with numbers and more to do with how scientists listen to the brain.
When the team examined earlier studies, they noticed that many analyses lumped all electrical activity together, without distinguishing between rhythmic oscillations (like the beta waves) and non-rhythmic background noise. Yet these two types of activity reflect very different neural processes.
“You can imagine the brain as a concert hall full of musicians,” says Moritz Gerster, who led the study. “Some groups are playing in rhythm, producing a clear, coordinated melody. Others are tuning their instruments or practicing independently, creating a background of random sounds. If you only measure the overall volume, you miss the difference between the music and the noise.”
By developing advanced analysis techniques, the researchers were able to separate the rhythmic beta oscillations from the non-rhythmic “neural noise.” When they did, the picture became much clearer. The rhythmic signals correlated strongly with movement difficulties, while the non-rhythmic background reflected other aspects of neural activity, possibly linked to overactive or misfiring neurons.
This distinction didn’t just clarify the data—it opened a new window into the biology of Parkinson’s disease itself.
Where the Rhythm Begins
The refined analysis also revealed that the rhythmic beta activity originated precisely from the same brain regions where deep brain stimulation proved most effective. This alignment suggests that the oscillations themselves may be direct targets for therapeutic intervention.
Currently, DBS systems are manually programmed by clinicians. Specialists adjust the electrode’s settings based on observation and experience, a process that can take weeks or months to fine-tune. But if doctors could map the brain’s abnormal rhythms in real time, they might be able to identify the optimal stimulation points automatically—an advance that could make DBS treatment faster, safer, and more consistent.
This finding could pave the way for intelligent, adaptive brain stimulators capable of responding dynamically to a patient’s neural activity. Instead of delivering constant pulses, these “smart” systems could activate only when abnormal rhythms emerge—restoring balance to the brain’s symphony precisely when it begins to falter.
Making Every Patient Their Own Control
Another major challenge in Parkinson’s research is the diversity of the patients themselves. No two people experience the disease in exactly the same way. Age, genetic background, duration of illness, and symptom profile all vary widely, making it difficult to compare results across groups.
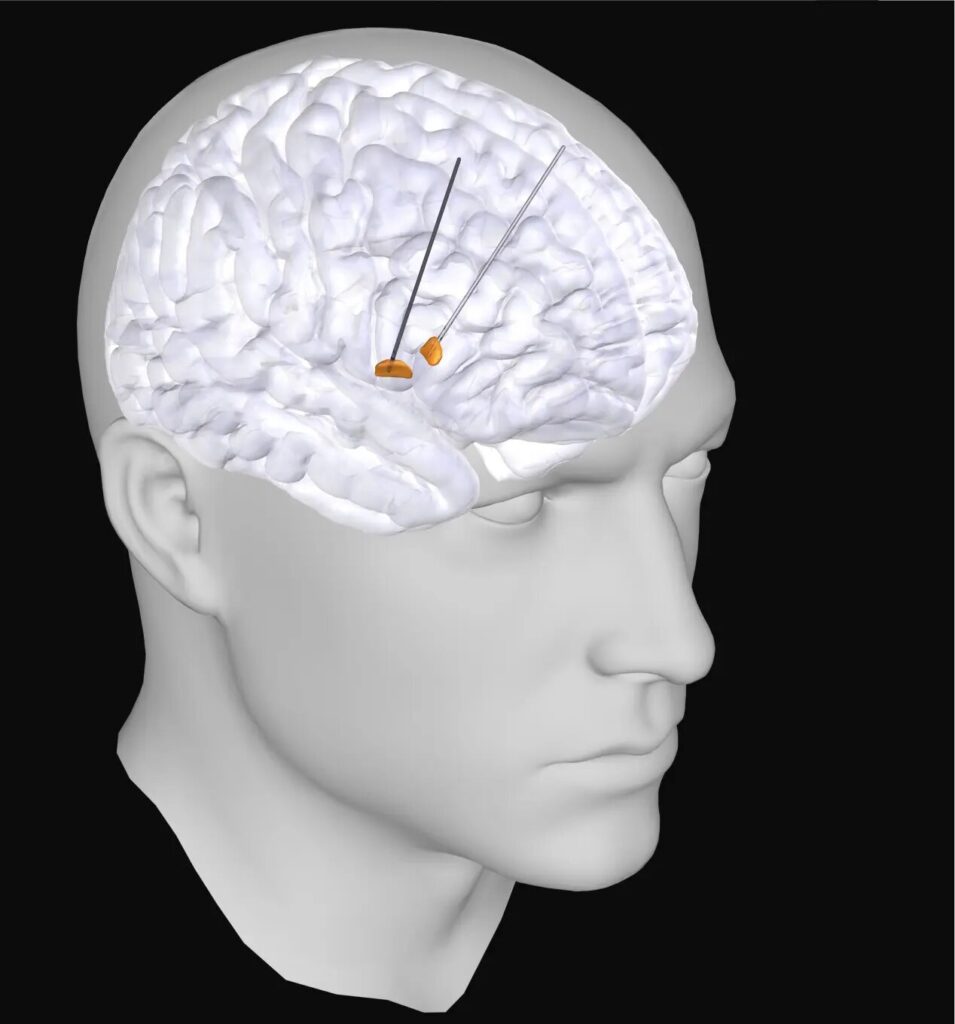
Moreover, ethical and practical limits prevent researchers from implanting electrodes in healthy individuals, which means there’s no straightforward control group for direct comparison.
The Max Planck-led team found a creative way around this. Parkinson’s disease typically affects one side of the body more strongly than the other. The researchers realized they could use this asymmetry as a built-in control mechanism. By comparing electrical activity between the more-affected and less-affected sides of the same patient’s brain, they could isolate disease-related changes while keeping individual differences constant.
This clever approach revealed another striking pattern. The more-affected hemisphere showed significantly higher levels of non-rhythmic, noise-like activity—suggesting an increase in the firing rate of neurons. This aligns with findings from animal studies, reinforcing the theory that overactive, uncoordinated neuronal firing contributes to Parkinson’s movement symptoms.
Toward Smarter, More Personalized Treatments
The discovery of this distinctive electrical “signature” could transform the future of deep brain stimulation. If clinicians can detect the telltale noise patterns and rhythmic disturbances in real time, they could tailor stimulation to the brain’s ongoing state.
Such adaptive DBS systems already exist in prototype form. They continuously monitor brain signals and adjust stimulation parameters automatically. The new findings from this study could help refine these systems—teaching them to recognize the specific neural signatures that accompany worsening symptoms.
In practice, that means a stimulator could quiet the brain’s abnormal activity exactly when needed, reducing side effects and conserving battery power. Patients would no longer receive continuous pulses, but dynamic, responsive therapy tuned to their unique neural rhythms.
A Model for Future Collaboration
Beyond its scientific achievements, this study represents something rare and inspiring: true international collaboration in a complex and competitive field. The researchers brought together hospitals and institutions across Germany and the United Kingdom, harmonizing their data and methods to achieve a common goal.
In neuroscience, where data collection is difficult and often proprietary, such cooperation is far from easy. Yet it proved essential here. By sharing expertise and resources, the team created a dataset large enough to reveal patterns invisible to smaller efforts.
Their success offers a powerful model for the future of medical research—one where openness and collaboration replace fragmentation, and where breakthroughs arise not from isolated labs but from united scientific communities.
The Promise of Understanding
At its heart, this research is about more than electrical signals or statistical correlations. It’s about understanding the human experience of Parkinson’s disease—the daily struggle to move, to speak, to live with independence. Each data point represents a person whose life is profoundly affected by the failure of their brain’s delicate rhythms.
The study’s findings don’t yet offer a cure. But they bring us closer to a world where therapy is not only more effective but also more personal—guided by each patient’s unique neural fingerprint. By learning to listen more carefully to the brain’s music, scientists are beginning to tune its instruments again, restoring some of the harmony that Parkinson’s disease takes away.
Looking Ahead
The next steps are already underway. Researchers will now test whether these neural signatures remain stable in everyday conditions—outside the controlled environment of the lab—and whether adaptive DBS can respond effectively to them in real time.
If successful, these advances could redefine how Parkinson’s disease is treated, transforming deep brain stimulation from a steady pulse into a dynamic conversation between technology and the living brain.
For millions of people around the world living with Parkinson’s, that conversation could mean something extraordinary: a future where their movements, once frozen or tremulous, begin to flow again with the grace of a restored rhythm—proof that even in the midst of neurological disorder, the brain’s music can find its harmony once more.
More information: Moritz Gerster et al, Beyond beta rhythms: subthalamic aperiodic broadband power scales with Parkinson’s disease severity–a cross-sectional multicentre study, eBioMedicine (2025). DOI: 10.1016/j.ebiom.2025.105988