Neuroblastoma is one of the most heartbreaking cancers in children. Striking mostly under the age of five, it grows from the nerve cells of the sympathetic nervous system and can appear in many parts of the body. The disease is unpredictable: in about half of all cases, tumors shrink on their own, almost like a cruel magic trick of nature. In the other half, they grow aggressively, responding well to chemotherapy at first — only to return with devastating force one to two years later.
At the center of this aggressiveness is a powerful cancer-driving gene: MYCN. Children with tumors containing unusually high numbers of MYCN copies often face the most dangerous forms of the disease. But now, scientists have discovered that where MYCN is located inside a cancer cell — not just how much of it there is — could determine whether the tumor can be stopped.
The Discovery That Changes the Game
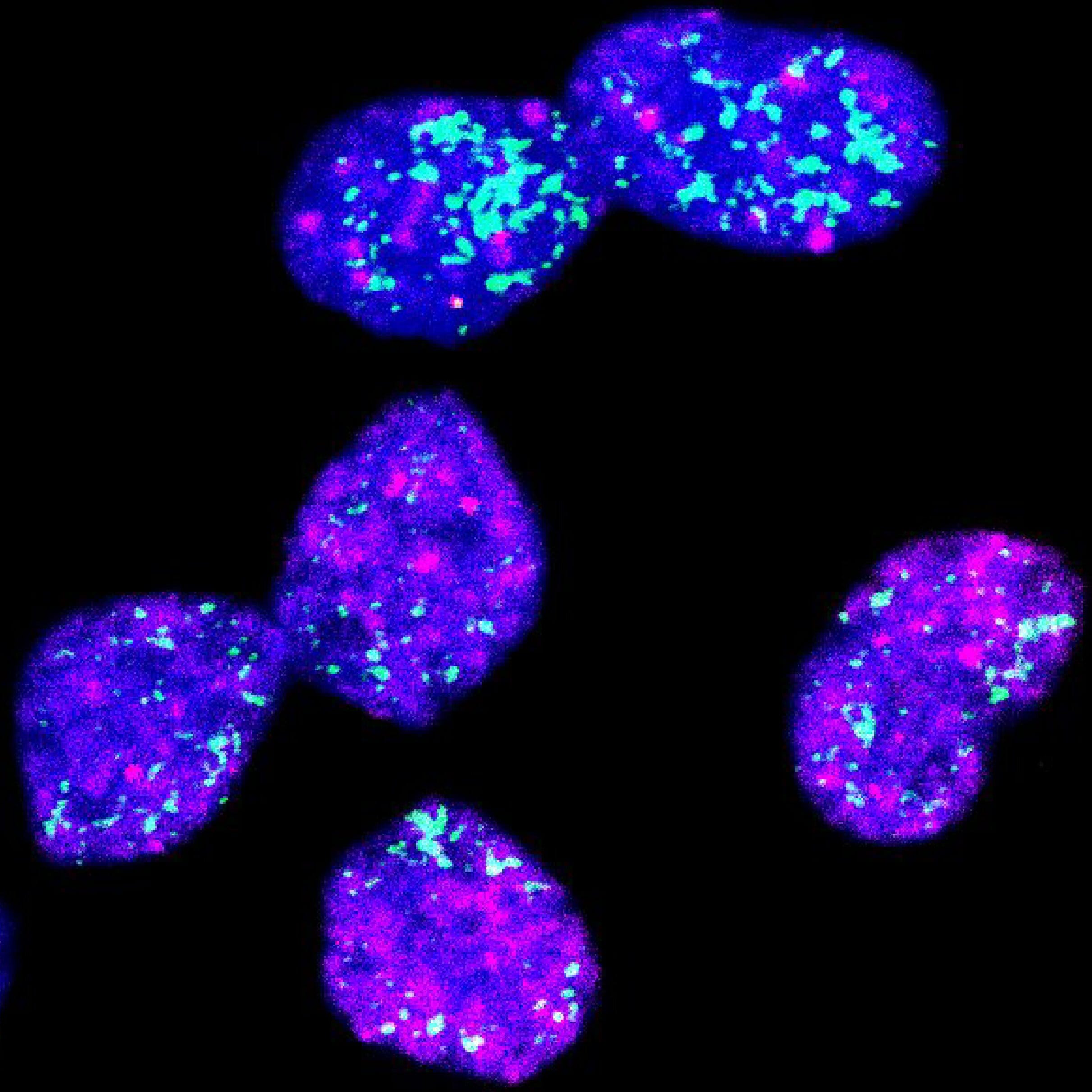
In a study published in Cancer Discovery, a team led by Dr. Jan Dörr and Professor Anton Henssen from the Experimental and Clinical Research Center (ECRC) — a joint institution of Charité—Universitätsmedizin Berlin and the Max Delbrück Center — revealed that MYCN sometimes exists outside chromosomes, on tiny, ring-shaped DNA fragments called extrachromosomal DNA. This unusual location can trigger an unexpected survival tactic in the cancer.
“When MYCN is on these little DNA rings instead of chromosomes, cancer cells can enter a kind of hibernation,” explains Dörr. “They stop growing, which sounds good at first — but it actually makes them invisible to standard therapies like chemotherapy.”
This dormant state effectively turns these cancer cells into sleeping enemies. They are not destroyed, just waiting, hidden from drugs that target rapidly dividing cells. At some unpredictable moment, they can “wake up” and fuel a relapse.
DNA Rings: The Cancer’s Secret Weapon
Henssen’s earlier work had shown that oncogenes — cancer-causing genes like MYCN — are often found on extrachromosomal DNA in pediatric tumors. These tiny DNA loops behave differently from chromosomes: when a cell divides, they are distributed randomly among daughter cells. This randomness creates a mixed population of tumor cells — some loaded with MYCN copies and others with very few.
The high-MYCN cells grow rapidly and are vulnerable to chemotherapy. The low-MYCN cells, however, can slip into deep sleep, or senescence. This creates a dangerous cycle: treatment wipes out the aggressive cells, but the sleepers remain, ready to reawaken and rebuild the tumor.
Hunting the Sleepers
To understand these sleeper cells, Dörr’s team developed a new method to separate tumor cells based on how many MYCN copies they carry. Working with Dr. Fabian Coscia’s Spatial Proteomics group, they studied the differences in proteins and behavior between the two cell types.
The results were clear: chemotherapy kills only the MYCN-rich cells. The rest survive — and because they are dormant, they resist virtually all standard treatments. “It’s like trimming the branches of a tree while leaving the roots untouched,” Dörr says. “Eventually, it grows back.”
A Two-Pronged Attack
Here’s where the breakthrough becomes hopeful. There are already drugs that specifically target senescent, or sleeping, cells — known as senolytics. The research team tested a combination approach in mice: first, standard chemotherapy to eliminate the fast-growing MYCN-heavy cells; then, a senolytic drug to kill the sleeping cells hiding in the background.
The results were striking. Tumors shrank more effectively and stayed away longer compared to chemotherapy alone.
“This is a completely new strategy,” says Dörr. “We’re not just going after the cancer we can see — we’re also destroying the seeds of its return.”
Beyond Neuroblastoma
The implications of this research go far beyond one childhood cancer. Many deadly tumors, including certain brain cancers, also carry oncogenes on extrachromosomal DNA. If similar dormant-cell behavior is found in these diseases, the same two-pronged therapy approach could save lives across cancer types.
“Our next step is to systematically search for more drugs that can target these dormant cells in human tissue while sparing healthy cells,” Henssen explains. “If we can perfect this approach, it could be transformative — not just for neuroblastoma, but for a range of aggressive cancers.”
A Model for Collaboration
This discovery is also a story about the power of global teamwork. The study involved scientists and clinicians from Berlin, London, and China, working under the international Cancer Grand Challenges initiative called eDyNAmiC. Pediatric oncologists, molecular biologists, and data scientists all contributed, turning a complex biological puzzle into a tangible new treatment idea.
“In pediatric oncology, time is precious,” says Dörr. “Every breakthrough that helps us understand why treatment fails brings us closer to cures that last.”
The Road Ahead
For families facing neuroblastoma, this discovery offers more than just scientific insight — it offers hope. Hope that relapses can be prevented. Hope that treatments can be designed to root out every hidden cell. And hope that the silent return of cancer can be stopped before it begins.
It will take more research, more clinical trials, and more international collaboration before this approach becomes a standard treatment. But for now, the message is clear: cancer’s ability to hide may no longer be its greatest strength.
And for the youngest patients, that could make all the difference.
More information: Giulia Montuori et al, Extrachromosomal DNA-Driven Oncogene Dosage Heterogeneity Promotes Rapid Adaptation to Therapy in MYCN-Amplified Cancers, Cancer Discovery (2025). DOI: 10.1158/2159-8290.CD-24-1738