For decades, people diagnosed with macular telangiectasia type 2 (MacTel) have faced a grim reality: a slow but relentless loss of central vision with no treatment to stop or even slow the disease. But a groundbreaking new therapy has changed that narrative — and it’s the result of years of persistence, international collaboration, and scientific ingenuity.
Researchers from Scripps Research, the National Institutes of Health (NIH), and Neurotech Pharmaceuticals have unveiled results from two major clinical trials showing that a surgically implanted device can significantly slow the progression of MacTel. The findings, published in NEJM Evidence, led to the U.S. Food and Drug Administration’s (FDA) approval of the device in March 2025. The treatment, known as ENCELTO (revakinagene taroretcel-lwey), is the first ever approved for MacTel — and the first cell-based neuroprotective therapy for any neurodegenerative retinal or central nervous system disease.
“This is a step toward redefining how we think about vision loss,” says Professor Martin Friedlander of Scripps Research, one of the study’s lead investigators. “Instead of waiting for cells to die, we’re learning how to protect and preserve them.”
How the Therapy Works
ENCELTO is more than just a medical device — it’s a miniature, living factory of neuroprotection. At its core are genetically modified retinal pigment epithelial cells, carefully engineered to release ciliary neurotrophic factor (CNTF), a naturally occurring protein known to protect light-sensitive neurons in the retina. These cells are sealed inside a tiny collagen-based capsule that is surgically placed in the back of the eye.
The capsule design is key. It shields the implanted cells from the patient’s immune system, preventing rejection, while allowing CNTF to diffuse steadily into surrounding retinal tissue. This constant, localized delivery bypasses the challenges of systemic treatments and ensures the retina receives a sustained therapeutic dose exactly where it’s needed.
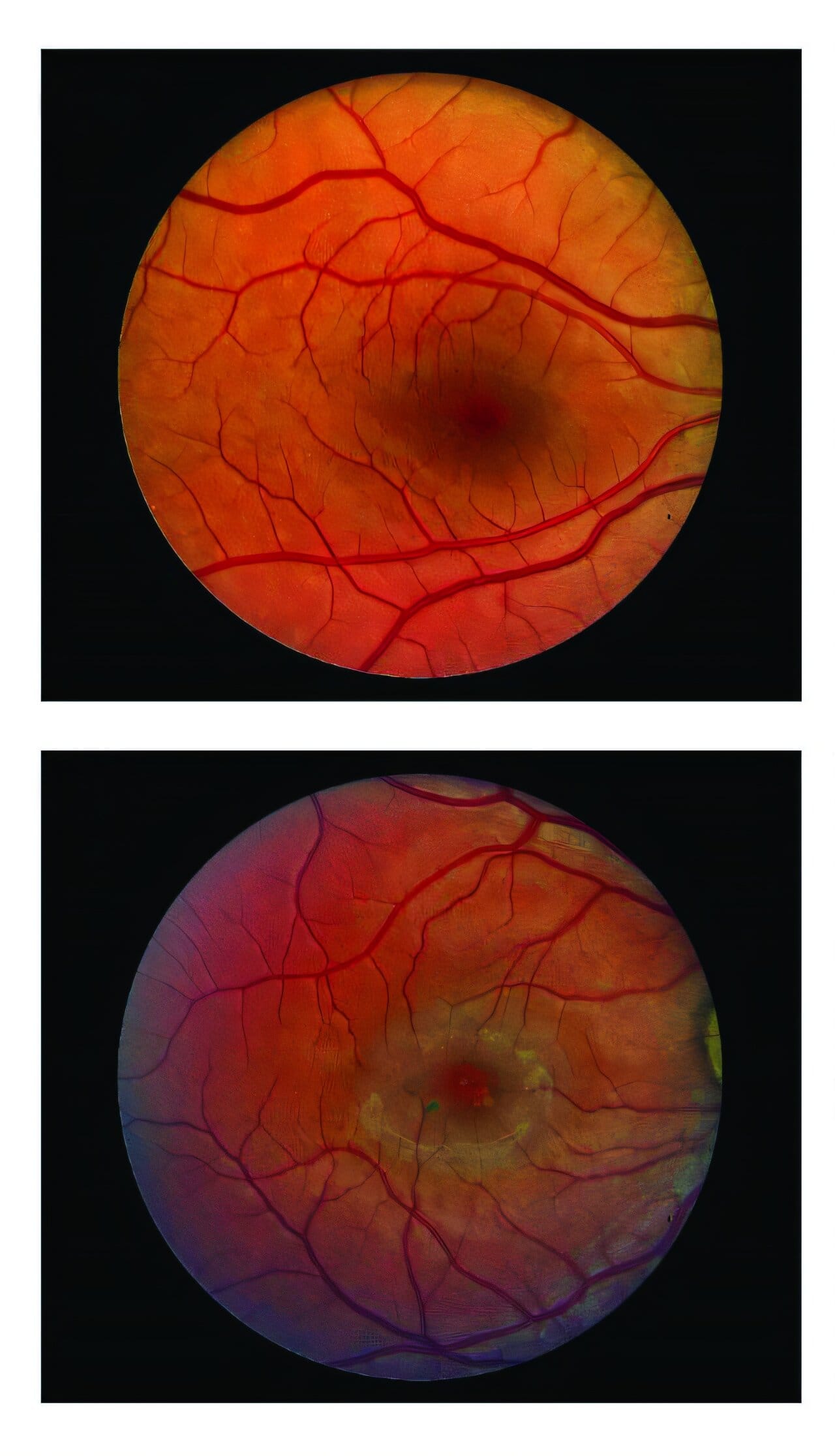
In MacTel, the central retina slowly loses photoreceptors — the specialized cells that detect light and enable sharp vision. By bathing these cells in CNTF, ENCELTO slows their degeneration, effectively preserving the eye’s “pixels” before they vanish forever.
The Landmark Clinical Trials
The FDA approval was supported by two Phase III clinical trials involving 228 participants across 47 sites worldwide. Each trial followed an identical design but recruited patients from different locations and at different times. Participants were randomly assigned to receive either the ENCELTO implant or a sham procedure that mimicked the surgery without delivering the therapy. Researchers then monitored structural changes in the retina and functional measures of vision over 24 months.
Both trials showed that ENCELTO slowed the loss of photoreceptors, although the effect size varied. In one trial, the implant reduced the rate of ellipsoid zone loss — a key indicator of photoreceptor degeneration — by 54.8% compared with sham-treated eyes. The second trial saw a 30.6% reduction, still statistically significant.
While structural preservation was clear in both studies, functional vision outcomes — such as reading speed and retinal sensitivity measured by microperimetry — showed mixed results. One trial demonstrated notable improvements, while the other found no statistically significant difference compared to the control group. When researchers combined data from both trials, however, the results strengthened, revealing a consistent benefit in slowing functional decline.
“These differences highlight just how complex it is to measure functional vision loss in a slow-progressing disease like MacTel,” Friedlander explains. “But the overall trend is unmistakable — preserving retinal cells preserves vision.”
A Decades-Long Scientific Journey
Friedlander, who is also president of the Lowy Medical Research Institute, has spent nearly twenty years studying MacTel’s biology. This groundwork laid the foundation for ENCELTO’s development, from understanding which retinal cells are vulnerable to identifying CNTF as a potent neuroprotective molecule.
The collaboration behind ENCELTO stretched across continents and disciplines, bringing together clinical ophthalmologists, retinal imaging specialists, molecular biologists, and bioengineers. The NIH’s National Eye Institute provided critical expertise, while Neurotech Pharmaceuticals contributed its proprietary encapsulated cell technology.
What This Means for Patients
The arrival of ENCELTO transforms the outlook for people living with MacTel. For the first time, there is an option to slow vision loss rather than simply watch it progress. The therapy appears to work regardless of the patient’s baseline vision or disease stage, though earlier treatment may preserve more functional vision over time.
Side effects from the implant were minimal, and patients generally tolerated the procedure well. Importantly, the therapy doesn’t require repeated injections or ongoing drug infusions — a single implant can continuously release CNTF for years.
“This isn’t just a treatment for MacTel,” says Friedlander. “It’s proof that we can intervene early in neurodegenerative eye diseases and preserve vision for longer.”
A Platform for the Future
Beyond MacTel, ENCELTO’s approach could pave the way for treatments targeting other blinding disorders caused by nerve cell loss. Because the implant can be engineered to release different therapeutic proteins, it could, in theory, be adapted to conditions like retinitis pigmentosa, glaucoma, or even certain optic nerve diseases.
“ENCELTO represents a versatile delivery platform,” Friedlander notes. “The retina is part of the central nervous system, so this technology could be a stepping stone to neuroprotection strategies for other degenerative conditions in the brain and spinal cord.”
Looking Ahead
The research team plans to follow patients beyond the initial 24 months to determine whether benefits persist or improve over time. They also aim to uncover why some patients respond more strongly than others, potentially guiding personalized treatment strategies in the future.
For now, the message is clear: protecting vulnerable cells before they die is not only possible but clinically achievable. For people with MacTel — and potentially millions more with other neurodegenerative eye diseases — that marks the beginning of a new era in vision care.
As Friedlander reflects, “The goal isn’t just to slow the clock on vision loss. It’s to give people more years of meaningful sight — to keep them reading, driving, recognizing faces, and living fully. That’s what makes this breakthrough so powerful.”
More information: Emily Y. Chew et al, Cell-Based Ciliary Neurotrophic Factor Therapy for Macular Telangiectasia Type 2, NEJM Evidence (2025). DOI: 10.1056/EVIDoa2400481