In the world of neurodegenerative diseases, few names carry as much weight as Parkinson’s disease. For millions across the globe, it is a daily battle—one marked by tremors, stiffness, and the gradual erosion of movement and independence. But beneath these visible symptoms lies a deeper, molecular mystery: what exactly drives the destruction of brain cells in Parkinson’s disease?
Now, an international team of scientists, spearheaded by Professor Minee L. Choi of the Korea Advanced Institute of Science and Technology (KAIST), has uncovered a critical piece of that puzzle. Their breakthrough reveals that an obscure biological process—RNA editing—is far more influential than previously imagined, potentially opening the door to a completely new class of therapies for Parkinson’s disease.
The Silent Saboteur: α-Synuclein
At the heart of Parkinson’s disease lies a rogue protein: α-synuclein. This protein, normally found in healthy neurons, becomes dangerous when it misfolds and forms aggregates within brain cells. These toxic clumps trigger a cascade of cellular dysfunction, leading to the slow but relentless death of neurons. But it’s not just neurons that are affected. Other brain cells, particularly astrocytes—supportive, star-shaped glial cells—are also pulled into the fray, and their role in the inflammatory landscape of Parkinson’s is increasingly drawing attention.
While the accumulation of α-synuclein has long been established as a hallmark of Parkinson’s, this study zooms in on the domino effect it sets off in astrocytes and, remarkably, how this process is governed by changes not in the DNA itself, but in how RNA—the molecular messenger that carries instructions from DNA to proteins—is edited.
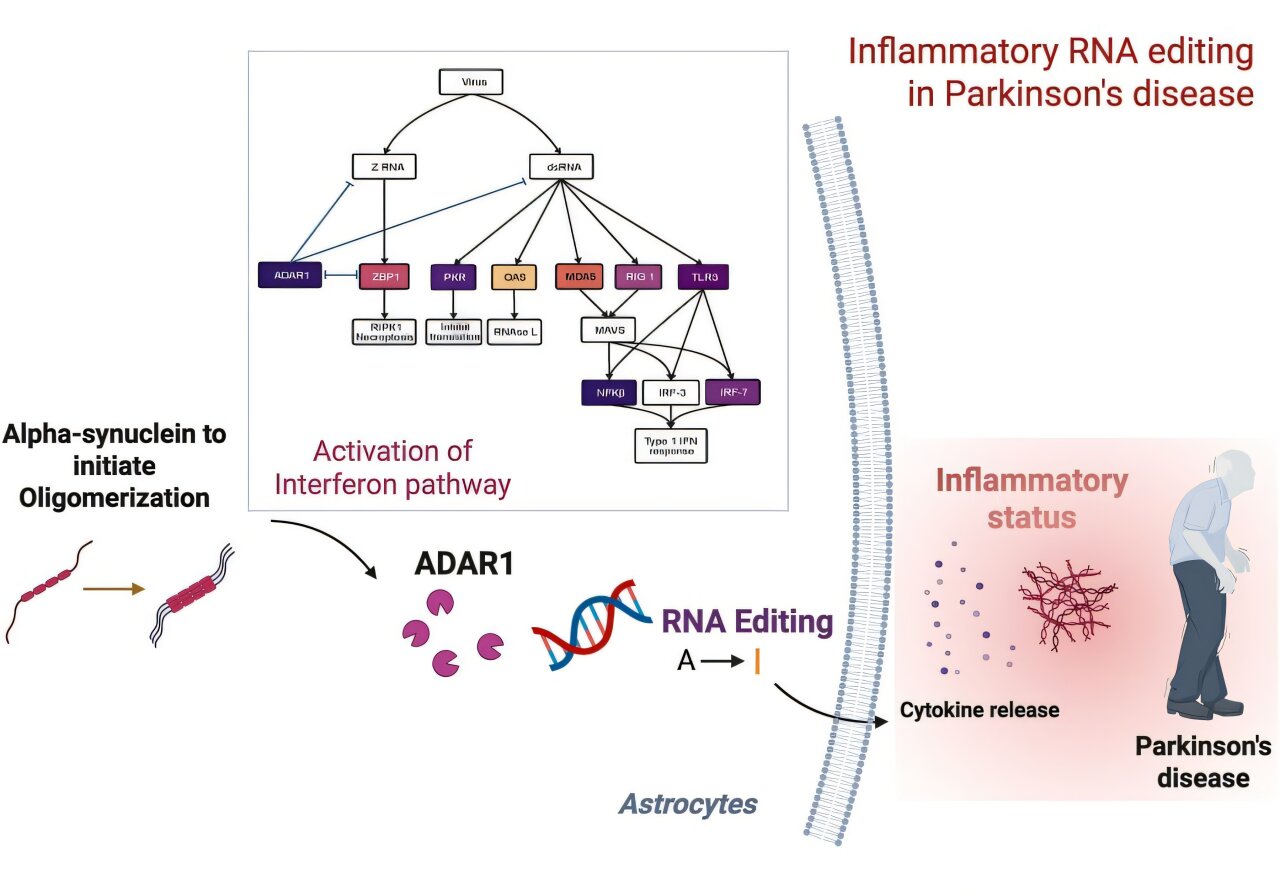
A Hidden Layer of Control: RNA Editing and ADAR1
RNA editing is a molecular sleight-of-hand. It subtly alters the sequence of RNA after it has been transcribed from DNA, often changing the resulting protein or its function. One of the key players in this process is an enzyme called ADAR1 (adenosine deaminase acting on RNA 1), which performs a very specific kind of editing: it changes adenosine (A) to inosine (I) in RNA strands, a mechanism known as A-to-I RNA editing.
This process is vital for normal immune regulation, especially in fighting off viral infections. But what happens when it goes awry?
Professor Choi and her collaborators from University College London (UCL) and the Francis Crick Institute discovered that in Parkinson’s disease, ADAR1 is no longer operating in its usual role. Instead of calmly keeping immune responses in check, it begins to misfire—editing the RNA of inflammation-related genes inappropriately, and thus fanning the flames of chronic brain inflammation.
Peering Into the Brain: Building a Disease in a Dish
To investigate this surprising mechanism, the researchers developed a pioneering lab model. They used induced pluripotent stem cells (iPSCs) derived from actual Parkinson’s patients to create a co-culture system—an experimental model in which neurons and astrocytes grow side by side. This model mirrors the real cellular environment of the human brain more closely than traditional approaches, and crucially, it reflects the personalized disease features of each patient.
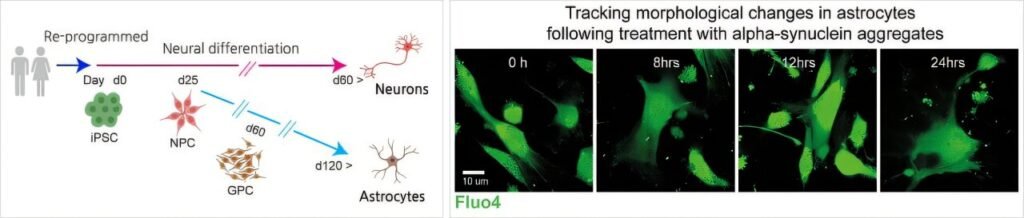
Into this model, they introduced α-synuclein oligomers—early toxic forms of the protein that are particularly known to spark inflammation. What they saw was dramatic. The astrocytes, usually the guardians of brain homeostasis, began to behave as if under siege. Their Toll-like receptor pathways—a kind of molecular danger alarm—were activated, as was the interferon response system, a defensive network more commonly associated with fighting viruses.
And it was here, in the middle of this cellular chaos, that ADAR1 stepped in. But it wasn’t the ADAR1 scientists knew. It had transformed into a different isoform—a version of the protein with altered structure and function. Instead of editing in a balanced, regulatory way, this altered ADAR1 began targeting inflammation-related genes with unusual intensity. The editing was no longer precise—it was reckless, amplifying immune responses and driving prolonged inflammation in astrocytes.
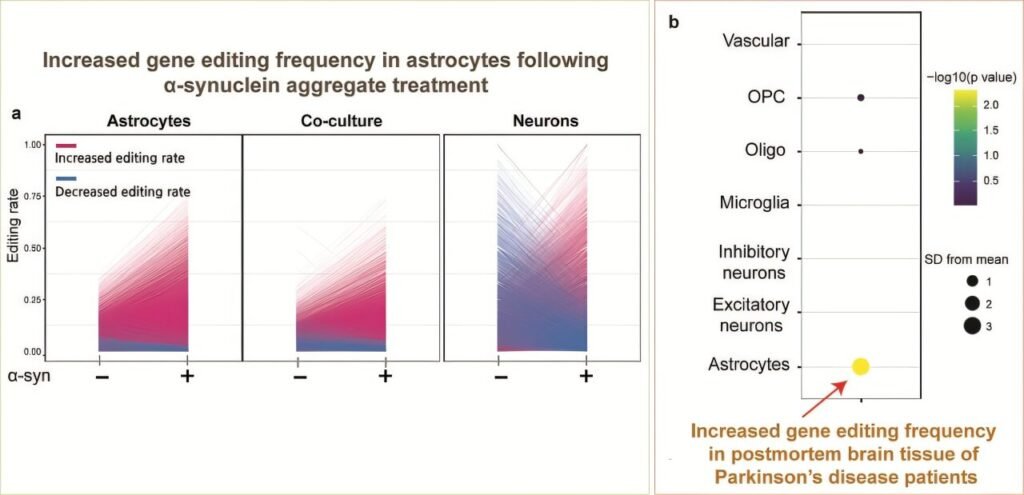
From Cells to Brains: Evidence in Human Tissues
To validate their findings, the team went beyond lab-grown cells. They analyzed postmortem brain tissues from patients who had died with Parkinson’s disease and found the same abnormalities in RNA editing. The pattern was unmistakable: the misregulation of ADAR1 and the resulting inflammatory response was not just an artifact of the lab—it was happening in real human brains.
This revelation is crucial. It means that RNA editing, once considered a niche area of molecular biology, is not only active in the human brain but directly involved in the pathology of Parkinson’s disease. It acts like a switchboard operator gone rogue—misdirecting signals and keeping the immune system in a state of perpetual, damaging alert.
Rethinking Parkinson’s Disease: A New Frontier in Therapy
What makes this discovery so exciting is that it shifts the spotlight onto a previously underexplored avenue for therapeutic intervention. Traditional Parkinson’s treatments have largely focused on managing symptoms—particularly the dopamine deficiency that results from the death of neurons in the brain’s motor centers. While these treatments can be life-changing, they do not halt or reverse the underlying disease process.
The identification of ADAR1’s role in astrocyte-mediated inflammation opens up the possibility of targeting RNA editing itself. Could we correct the editing errors? Could we modulate ADAR1’s function to reduce inflammation without compromising its normal roles? These questions are now at the forefront of a new chapter in neurodegenerative disease research.
As Professor Choi puts it, “This study demonstrates that the regulator of inflammation caused by protein aggregation operates at the new layer of RNA editing, offering a completely different therapeutic strategy from existing approaches to Parkinson’s disease treatment.” It’s a strategy that doesn’t aim to clear α-synuclein aggregates directly or simply replenish lost dopamine—but rather, to quiet the chronic, smoldering immune response that may be driving much of the damage.
Personalized Models, Precision Insights
Another standout aspect of this study is the use of patient-derived iPSC models. These allow scientists to recreate disease processes using the actual genetic and molecular signatures of individual patients. It’s a profound step toward precision medicine—where therapies can be tailored not just to diseases, but to people.
By using cells from real Parkinson’s patients, the team ensured that their findings reflect the messy, complex reality of the human brain—not just idealized models. And the fact that they replicated their findings in postmortem tissue further strengthens the case for RNA editing as a major player in Parkinson’s pathology.
The Broader Implications: Beyond Parkinson’s
While this study centers on Parkinson’s disease, the implications reach much further. Neuroinflammation is a common thread in many neurodegenerative disorders, including Alzheimer’s, Huntington’s, and amyotrophic lateral sclerosis (ALS). If RNA editing and ADAR1 are similarly involved in those conditions, we may be looking at a universal mechanism—a master regulator of immune responses in the brain that, when dysregulated, unleashes destruction.
This also raises tantalizing questions about the potential of RNA editing technologies, which are rapidly advancing. Could we design molecular tools to correct specific RNA edits in astrocytes? Could this approach offer a safer, more flexible alternative to genome editing? These are no longer far-off fantasies but emerging areas of real research and development.
A Turning Point in Parkinson’s Research
In the grand story of Parkinson’s disease, this discovery is a milestone. It does not offer an immediate cure—but it does offer a new narrative. One in which astrocytes are not mere bystanders but active participants in disease progression. One in which RNA editing, long relegated to the scientific sidelines, steps into the spotlight as a potential key to halting neurodegeneration.
It’s a story that highlights the power of international collaboration, of patient-centered research, and of thinking outside the traditional boxes of disease mechanisms. And at its heart is a reminder that the brain is not just a collection of neurons—it is an ecosystem, and when its immune balance is disturbed, the consequences can be profound.
As scientists continue to unravel the complexities of Parkinson’s, discoveries like this one are charting new paths toward a future where we don’t just treat the symptoms of brain diseases—we understand them at their roots and intervene with precision and purpose.
Reference: Karishma D’Sa et al, Astrocytic RNA editing regulates the host immune response to alpha-synuclein, Science Advances (2025). DOI: 10.1126/sciadv.adp8504