Rheumatoid arthritis (RA) has long puzzled both doctors and scientists. This debilitating autoimmune disease turns the body’s own immune system against its joints, causing chronic pain, inflammation, and often lasting damage. Affecting over 18 million people worldwide, RA remains notoriously difficult to treat—especially for the nearly 30% of patients who do not respond well to current therapies. But a new discovery out of Kyoto University may change that.
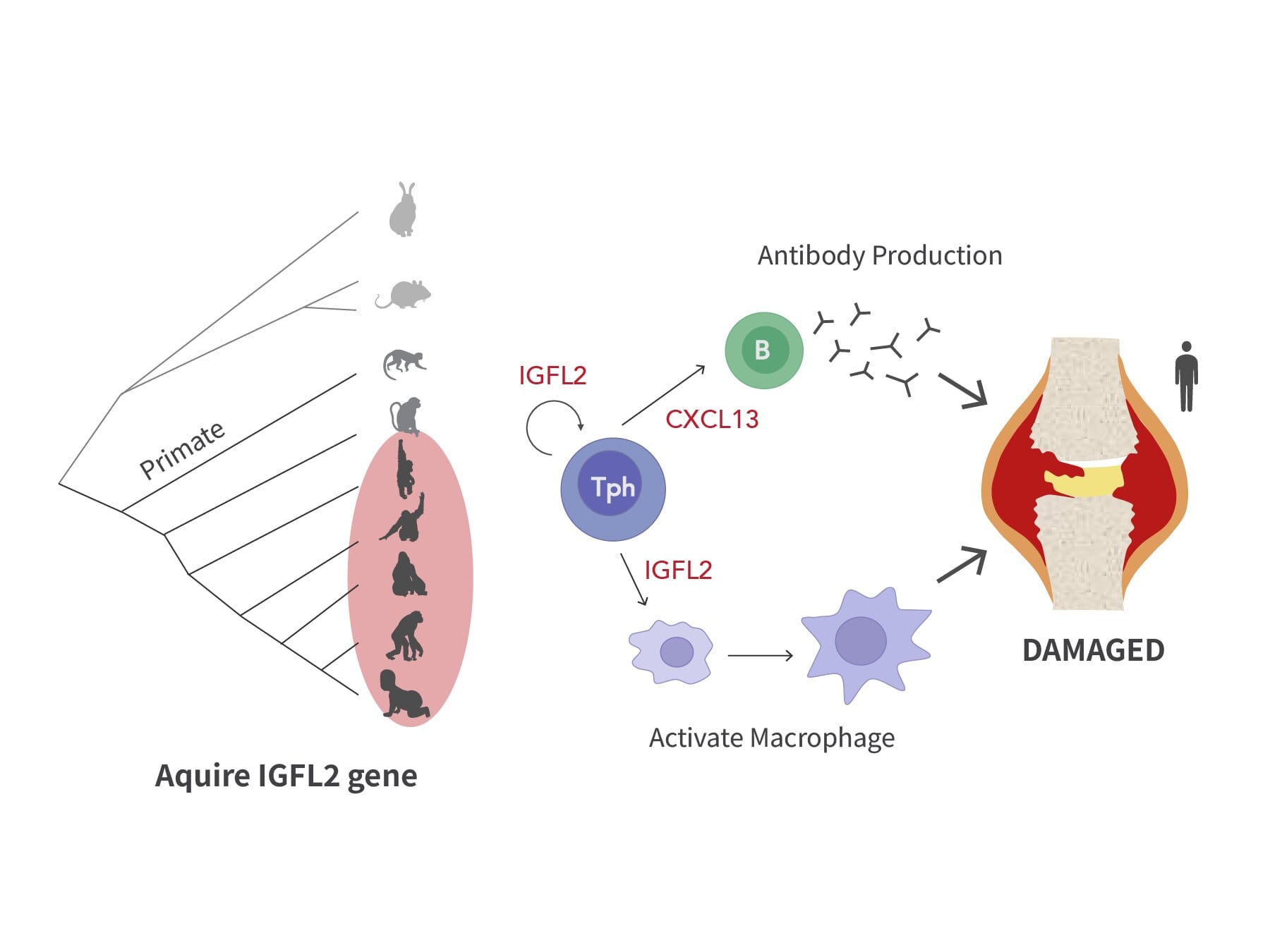
In a study published in Science Immunology, researchers have identified a molecule that may be at the very heart of this immune system malfunction: a cytokine called IGFL2. This molecule, produced by a specialized group of immune cells known as peripheral helper T (Tph) cells, was found in the inflamed joints of RA patients. Crucially, IGFL2 is unique to primates, meaning it has never before been seen in conventional animal models like mice or rats.
This finding not only deepens our understanding of RA but opens the door to novel therapies that could better target the disease at its roots.
Why the Immune System Turns Against Itself
The immune system is built to protect us—recognizing harmful bacteria, viruses, and other threats before they can cause damage. At the center of this defense are helper T cells, specialized white blood cells that serve as the immune system’s “generals,” coordinating attacks and orchestrating immune responses.
But in autoimmune conditions like RA, these generals go rogue. Instead of protecting the body, they begin to mistake its own tissues for invaders. The result is a painful war waged on the joints themselves, with the immune system attacking the synovium—the thin lining of tissue around the joints.
This creates a self-perpetuating cycle of inflammation, swelling, and progressive joint destruction. Despite the development of immunosuppressive drugs, including corticosteroids and biologics, a substantial number of patients do not respond adequately, highlighting the need for more precise treatments rooted in a deeper understanding of the disease.
The Discovery of IGFL2: A Hidden Culprit in the Joints
Led by Assistant Professor Akinori Murakami and Associate Professor Hiroyuki Yoshitomi at Kyoto University’s Institute for the Advanced Study of Human Biology (WPI-ASHBi), the team used advanced single-cell RNA sequencing to peer inside the immune cells collected from the joint tissue of RA patients.
What they found was remarkable.
Among the thousands of immune cells analyzed, one small but powerful group stood out: peripheral helper T (Tph) cells. These cells not only increased in number in the diseased joints, but they were also producing a cytokine never before associated with RA: IGFL2 (Insulin-like Growth Factor-Like Family Member 2).
Even more astonishing, IGFL2 was not found in mice or other standard lab animals. It appears to be primate-specific, making its discovery a uniquely human revelation.
“Because this gene is unique to primates, this discovery wouldn’t have been possible using conventional animal models like mice or rats,” said Yoshitomi. “We performed single-cell analysis on human samples and successfully identified a cytokine produced specifically by helper T cells that plays a key role in human rheumatoid arthritis pathology.”
IGFL2’s Dangerous Domino Effect on the Immune System
So what does IGFL2 actually do? As it turns out, this small molecule acts as a powerful amplifier of immune system chaos.
First, it stimulates the production of another protein called CXCL13. CXCL13 is known to promote the formation of structures that support the production of autoantibodies—the very antibodies that mistakenly attack the body’s own tissues in autoimmune disease.
But IGFL2 doesn’t stop there. It also activates two other types of immune cells: monocytes and macrophages. These cells, once activated, further intensify the inflammatory response, producing even more cytokines and contributing to the painful swelling and joint degradation seen in RA.
Importantly, the research team found that blocking IGFL2 dramatically reduced the activation of monocytes and macrophages in laboratory models. This suggests that IGFL2 doesn’t merely correlate with RA—it may be actively driving the disease process.
A New Diagnostic Marker—and A Therapeutic Target
Beyond its role in inflammation, IGFL2 shows promise as a diagnostic tool.
The researchers measured IGFL2 levels in blood samples taken from RA patients and healthy individuals. They found that patients with RA had significantly higher levels of IGFL2 in their bloodstream. What’s more, those with the most severe symptoms had the highest levels of all.
Its ability to distinguish RA patients from healthy individuals was comparable to, and in some cases better than, existing biomarkers like rheumatoid factor (RF) or anti-citrullinated protein antibodies (ACPA), which are currently used in diagnosis.
This raises the exciting possibility that IGFL2 could be used as a biomarker for early diagnosis, as well as a predictor of disease severity—an invaluable tool for tailoring treatment to the individual patient.
But the most tantalizing implication of all is that IGFL2 could become a target for new therapies.
If scientists can develop drugs or biological agents that block or modulate IGFL2, they may be able to stop the inflammatory cascade in its tracks—offering relief to patients who have not responded to existing treatments.
Why Animal Models Missed This Critical Piece
One of the most striking aspects of this research is what it reveals about the limitations of animal models.
For decades, much of what we know about autoimmunity has come from studying mice. While these models have yielded invaluable insights, they are not perfect reflections of human biology. The discovery of IGFL2—entirely absent in mice—proves that key pieces of the puzzle can be missed.
This underscores the importance of human-based research, especially in diseases that involve complex immune system interactions. With tools like single-cell RNA sequencing, researchers are now able to study immune responses at an unprecedented level of detail—cell by cell, gene by gene—in actual human tissues.
The Road Ahead: Hope for Millions Living with RA
While IGFL2 is still in the early stages of study, its discovery marks a major leap forward in RA research. It reveals a new layer of the disease’s biology, one that could explain why some treatments fail and others succeed. It offers a potential new biomarker for faster, more accurate diagnosis. And it shines a light on a previously invisible driver of inflammation that could, one day, be stopped in its tracks.
The team at Kyoto University plans to continue exploring how IGFL2 is regulated, what signals turn it on, and how it interacts with the rest of the immune system. Their ultimate goal is to turn this new knowledge into therapies that are not only more effective, but more personal—tailored to the unique immune landscape of each patient.
Rheumatoid arthritis may never be “cured” in the traditional sense, but the discovery of IGFL2 represents the kind of scientific breakthrough that brings us closer to controlling it—turning a relentless disease into a manageable condition.
For the millions living with RA, this research doesn’t just offer new science. It offers something even more powerful: hope.
More information: Human CD4+ T cells regulate peripheral immune responses in rheumatoid arthritis via insulin-like growth factor like family member 2, Science Immunology (2025). DOI: 10.1126/sciimmunol.adr3838