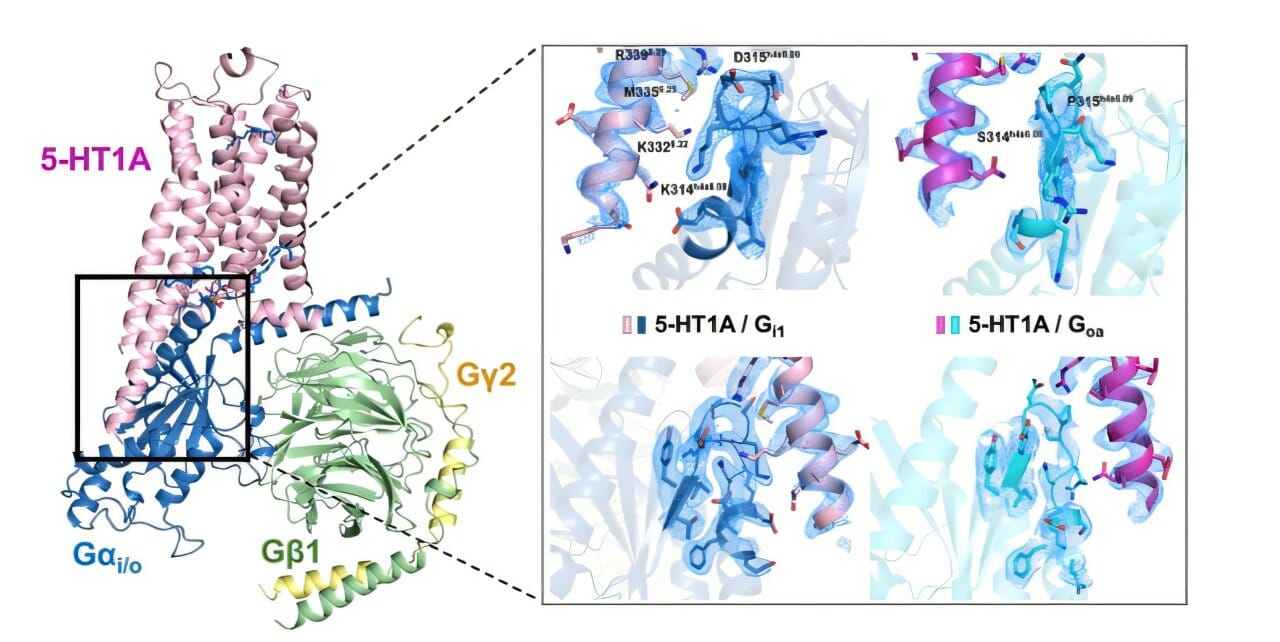
In a breakthrough that could reshape how doctors treat depression, anxiety, schizophrenia, and even chronic pain, scientists at the Icahn School of Medicine at Mount Sinai have revealed the inner workings of one of the brain’s most important serotonin receptors. Published in Science Advances, the study gives scientists an unprecedented look at how the 5-HT1A receptor—a critical “control panel” for mood regulation—operates at the molecular level.
Though long recognized as a target of antidepressants and psychedelic therapies, the 5-HT1A receptor has remained frustratingly elusive in terms of its structure and exact function. This new research, however, doesn’t just scratch the surface. It dives deep into the receptor’s internal logic, illuminating how drugs interface with it, how it filters incoming signals, and—perhaps most intriguingly—how a hidden molecule embedded in the cell membrane may steer its behavior like a biochemical co-pilot.
The findings offer new hope for developing next-generation psychiatric medications that act more quickly, more accurately, and with fewer side effects than anything available today.
The Master Switch of Mood and Emotion
To understand why this discovery matters, it helps to grasp what serotonin is—and what it isn’t. Often dubbed the “feel-good” chemical, serotonin is far more than a happiness hormone. It regulates mood, sleep, appetite, cognition, and even how we experience pain. It works by binding to receptors on neurons, effectively passing along chemical messages that help brain cells communicate.
The 5-HT1A receptor is one of the most crucial serotonin receptors in the brain. Imagine a control panel that helps determine how loudly or softly the serotonin signal is broadcast. It doesn’t just amplify or mute mood—it fine-tunes emotional response, perception, and psychological stability.
“This receptor is like a control panel that helps manage how brain cells respond to serotonin, a key chemical involved in mood, emotion, and cognition,” explains senior study author Dr. Daniel Wacker, Assistant Professor of Pharmacological Sciences and Neuroscience at Mount Sinai.
But until now, that panel remained stubbornly difficult to read, let alone manipulate with precision.
How the Brain Decides What to Hear
Using cryo-electron microscopy (cryo-EM)—an ultra-high-resolution imaging technique—the research team mapped the 5-HT1A receptor down to nearly atomic detail. Their goal was simple but profound: figure out how different drugs influence the receptor’s behavior.
It turns out that the receptor doesn’t treat all drugs the same. Instead, it shows a natural preference for certain internal signaling routes, known as G protein pathways. These pathways are like downstream wires, carrying messages from the receptor to the rest of the neuron.
“Some drugs amplify certain pathways while barely touching others,” says Dr. Audrey L. Warren, the study’s lead author and a postdoctoral researcher at Columbia University. “That’s a big deal because each pathway influences different brain functions—like mood, anxiety, or pain. If we can selectively activate only the beneficial ones, we could drastically reduce side effects.”
One striking example is asenapine (Saphris), an antipsychotic used to treat schizophrenia and bipolar disorder. The researchers found that asenapine selectively interacts with just one of the receptor’s signaling paths, hinting that its effectiveness—and limitations—may stem from this focused activity.
The Unexpected Role of a “Hidden Co-Pilot”
But the biggest surprise came not from the receptor itself, nor from the drugs that bind to it—but from the membrane fat surrounding it. Among the thousands of images the team analyzed, they noticed that a phospholipid—a type of fatty molecule that makes up the cell membrane—was playing an unexpected role.
Instead of passively surrounding the receptor, the lipid seemed to influence its shape and activity, acting almost like a molecular co-pilot guiding its behavior.
“This is the first time such a direct role has been observed in any of the 700-plus receptors in this family,” Dr. Wacker says. “It’s like discovering that the cockpit of an airplane is being partially steered by the dashboard’s design, not just the pilot. That changes how we think about drug design.”
This discovery hints at a new layer of drug targeting: not just hitting the receptor itself, but also tweaking the cellular environment around it to fine-tune its responses.
Toward Faster-Acting and More Precise Therapies
For millions of people battling depression or anxiety, existing treatments can be frustratingly slow. Standard SSRIs (Selective Serotonin Reuptake Inhibitors) often take weeks to become effective—if they work at all.
The Mount Sinai team’s findings may explain why.
If the receptor has built-in preferences for certain signaling routes, and those routes affect only part of the emotional circuitry involved in depression, then drugs that broadly increase serotonin might be too blunt to work quickly—or may even activate the wrong pathways.
“By understanding how this receptor functions at a molecular level, we have a clearer path to designing faster, more effective treatments,” says Dr. Wacker. “Not just for depression, but also for psychosis, PTSD, and even chronic pain. It’s a key piece of the puzzle.”
This is especially relevant in the emerging field of psychedelic-based medicine, where researchers are trying to isolate the therapeutic benefits of substances like psilocybin and LSD without triggering hallucinations. Knowing exactly which pathways matter—and how to activate them—makes this goal much more attainable.
What Comes Next: From Lab to Life
The team is now working to test whether their findings hold up in more complex biological systems, such as animal models and eventually human trials. They are also investigating how to translate this molecular map into real-world drug candidates—a process that involves chemistry, biology, and a little bit of luck.
“We’re designing molecules that can hit the receptor in just the right way, or that can nudge that lipid co-pilot into giving us a better outcome,” says Dr. Warren. “It’s like building a lock-and-key system, but now we understand not just the shape of the lock, but how it responds when you turn it.”
Their work builds on earlier successes from the same lab, which has previously created new classes of serotonin-modulating compounds derived from psychedelics but engineered for safety and specificity.
Hope on the Horizon for Mental Health Care
At a time when mental health disorders are rising and traditional treatments have plateaued, the importance of this discovery cannot be overstated. Depression is now the leading cause of disability worldwide, according to the World Health Organization. Anxiety and schizophrenia affect millions more, often with devastating personal and societal consequences.
“This research isn’t just about molecular biology—it’s about giving people their lives back,” says Dr. Wacker. “For someone who’s tried three or four medications and still feels hopeless, a more targeted, faster-acting therapy could mean everything.”
As science peels back the layers of the brain’s most important systems, one thing becomes clear: understanding how the smallest pieces work may offer the biggest hope.
What started as an obscure receptor buried in the cell membrane has now been thrust into the spotlight. With the tools of modern science, it has become a gateway—not just to better treatments, but to a deeper understanding of how we feel, think, and heal.
More information: Audrey Warren et al, Structural determinants of G protein subtype selectivity at the serotonin receptor 5-HT1A, Science Advances (2025). DOI: 10.1126/sciadv.adu9851. www.science.org/doi/10.1126/sciadv.adu9851