Imagine a world where the essential catalysts that drive life-changing chemical reactions—like those used to create fertilizers, pharmaceuticals, and energy—don’t depend on scarce, expensive, and often environmentally harmful metals. What if these vital chemical transformations could happen just as efficiently, but with elements that are both abundant and environmentally friendly? This could soon be a reality, thanks to a groundbreaking discovery made by researchers at The University of Osaka.
In a recent publication in the Journal of the American Chemical Society, the team revealed an exciting new reagent made from gallium, a main-group element, that displays the kind of reactivity previously thought to belong only to precious transition metals. This innovation not only opens the door to sustainable chemistry but could also change how we think about catalysts—chemicals that speed up reactions—in the future.
The Catalyst Conundrum
Catalysts are the unsung heroes behind many chemical processes that are fundamental to our modern world. They make reactions happen faster and more efficiently, without being consumed in the process. Whether it’s the production of life-saving medications, fertilizers that feed billions, or even the creation of clean energy, catalysts are essential.
Traditionally, many of the most effective catalysts have been based on transition metals, such as platinum, palladium, and rhodium. These metals are excellent at facilitating chemical reactions, especially those involving oxidation and reduction, commonly known as redox reactions. Redox reactions are vital in processes that produce everything from plastics to drugs. However, transition metals are not without their problems. They’re rare, expensive, and their extraction often harms the environment. Moreover, their availability is increasingly susceptible to geopolitical disruptions, making them unreliable in global supply chains.
But now, scientists have found a way to sidestep these limitations, thanks to a curious discovery made by a team of researchers in Japan.
Gallium’s Unexpected Role
At first glance, gallium, a group 13 element in the periodic table, might not seem like the perfect candidate to step into the spotlight as a redox reagent. Unlike transition metals, gallium and its neighboring elements in group 13 have never been known to participate in redox reactions in the same way. However, the team at The University of Osaka made a bold leap by testing gallium in this unfamiliar role—and the results were nothing short of revolutionary.
Their discovery shows that gallium, when used in an organic compound, can mimic the behavior of transition metals under visible light. Specifically, it can undergo a redox reaction—a crucial process for synthesizing complex molecules—when exposed to light. This is a significant breakthrough, as it opens up the possibility of using this earth-abundant metal to replace more expensive and ecologically damaging metals in chemical processes.
Lead author Nijito Mukai emphasized the significance of this breakthrough, stating, “There have been breakthroughs in using heavier group 14 and 15 elements in redox reagents for organic chemistry, but none using group 13 elements.” The gallium-based reagent they developed, he noted, is the first organic gallium compound to show this type of redox activity.
A New Path for Sustainable Catalysis
So why does this discovery matter? The implications are far-reaching. Gallium’s successful application as a redox reagent not only demonstrates its versatility but also marks a key step in the movement toward more sustainable chemistry. As we face increasing environmental and supply-chain challenges surrounding rare-earth metals, finding alternatives that are both more abundant and less harmful to the planet is critical.
“This is an important proof-of-concept that shows that group 13 elements can be used in redox reagents,” said senior author Takuya Kodama. “Overcoming the challenges of using a group 13 element will expand the use of main-group elements in redox catalysts.” By proving that gallium can function in this vital role, the researchers have paved the way for more widespread use of other common elements, such as aluminum, in similar chemical processes.
The potential benefits of this discovery go far beyond just gallium itself. It offers a new strategy for creating catalysts that rely on earth-abundant, low-cost materials, reducing our dependence on precious metals like palladium and platinum. This, in turn, could make important chemical processes not only more sustainable but also more accessible to industries around the world. Whether it’s in the production of drugs, energy, or materials for future technologies, gallium-based catalysts could transform entire sectors, making them greener and more resilient.
A Step Toward the Future
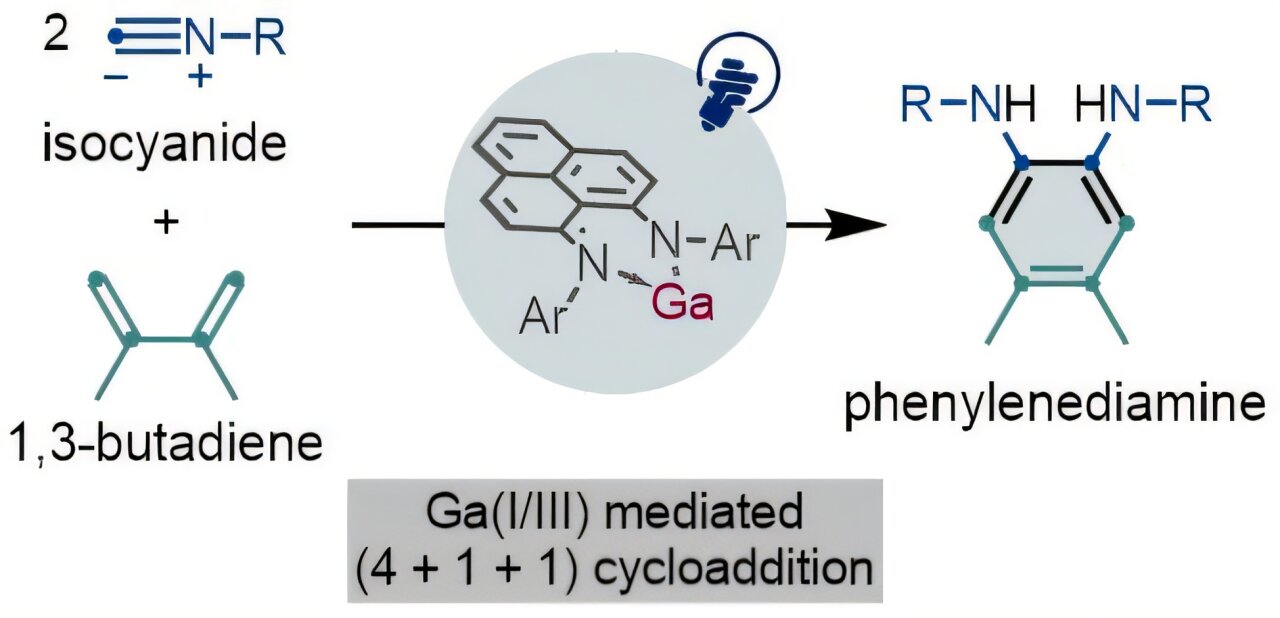
The immediate result of the Osaka team’s work is an efficient, visible-light-activated reaction that creates phenylenediamines—chemical building blocks used in pharmaceuticals and functional materials. This reaction, which forms an unusual ring structure, could become an essential tool in industries that rely on complex organic molecules. It’s a glimpse into a future where the chemistry behind medicine, energy, and materials might be fundamentally altered, thanks to a small but significant shift in how we think about the elements around us.
But the real promise of this discovery lies in its potential to catalyze a broader movement. As researchers continue to explore how main-group elements like gallium can take on the roles once reserved for expensive metals, we may see an entire new class of catalysts emerge—ones that are both environmentally friendly and economically viable. This shift could lead to more sustainable industrial practices, less reliance on rare metals, and the creation of more robust and affordable technologies.
Why This Research Matters
This discovery is much more than just a novel chemistry experiment—it’s a step toward a more sustainable and equitable future. As we continue to face challenges related to resource scarcity and environmental degradation, finding ways to replace expensive and environmentally damaging elements with more abundant, eco-friendly alternatives is crucial. The work of The University of Osaka team offers a promising glimpse into how we can achieve this.
Not only does this research reduce our dependence on transition metals, but it also has the potential to drive the next wave of scientific innovation. Whether it’s in the development of new medicines, clean energy technologies, or advanced materials, gallium’s unexpected role as a catalyst could help pave the way for breakthroughs that will benefit society in profound ways.
In the end, this breakthrough is about more than just chemistry—it’s about redefining what is possible in science and technology. By harnessing the power of an earth-abundant element like gallium, we might just be on the cusp of a new era in sustainable catalysis. One that doesn’t just change the way we do science, but transforms the world we live in.
More information: Synthesis of Phenylenediamines via (4+1+1) Photocycloaddition of 1,3-Dienes and Isocyanides Enabled by a Gallium(I)/(III) Redox: The Key Role of a Phenalenyl-Based Ligand, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c15802