Pancreatic cancer has long been one of the most feared diagnoses in medicine. Known for its relentless progression and resistance to treatment, it often hides quietly until it has already advanced beyond the point of cure. Pancreatic ductal adenocarcinoma, the most common form of the disease, is particularly ruthless. Fewer than 10% of patients survive five years after diagnosis—a stark reminder of its devastating toll.
What makes this cancer so difficult to treat is not only its late detection but also its unusual biology. Unlike many other tumors, pancreatic cancers are surrounded by an unusually dense, fibrous tissue called the stroma. This tissue acts like armor, forming a physical barrier that blocks drug delivery. Intuitively, such a barrier should also make it harder for cancer cells to escape into the bloodstream and spread to distant organs. Yet, paradoxically, pancreatic cancer metastasizes with alarming efficiency. For decades, researchers have asked: How does a cancer so heavily shielded manage to spread so quickly?
The Breakthrough Discovery
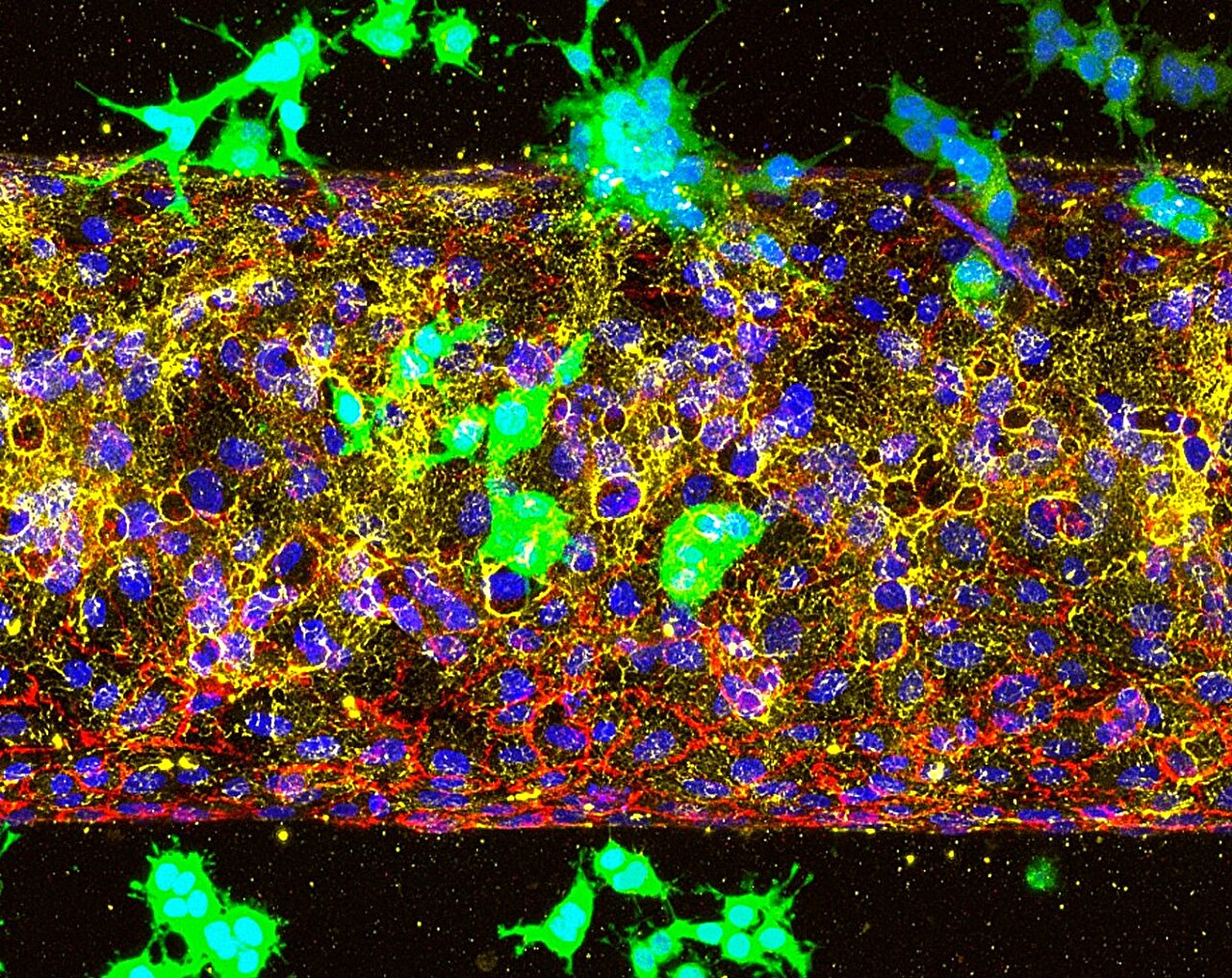
A team of scientists at Cornell University has now shed light on this puzzle, revealing the molecular trick pancreatic cancer uses to invade the bloodstream. In a study published in Molecular Cancer, they identified a receptor called ALK7 as a key driver of metastasis. Far from being a passive player, ALK7 is a master orchestrator, activating two distinct but interconnected pathways that together enable cancer cells to break free.
One of these pathways triggers a biological process known as epithelial-mesenchymal transition (EMT). Normally, this process allows cells to move during embryonic development or wound healing. But in cancer, EMT equips tumor cells with the mobility they need to detach from the primary tumor and begin their journey. The second pathway pushes the tumor’s invasion further, producing specialized enzymes that break down the walls of blood vessels.
“ALK7 gives pancreatic cancer cells both the engine to move and the tools to invade,” explained Esak Lee, lead author of the study and assistant professor in the Meinig School of Biomedical Engineering at Cornell Engineering.
Resolving a Scientific Mystery
ALK7 has been a controversial figure in cancer biology. In some studies, it appeared to act as a brake, slowing tumor spread. In others, it seemed to function as an accelerator. The Cornell study brings clarity to this confusion. By using advanced experimental systems, including mouse models and organ-on-chip technologies that mimic the architecture of human blood vessels, the researchers demonstrated conclusively that in pancreatic cancer, ALK7 drives metastasis rather than suppressing it.
When the team blocked ALK7 in these models, the results were striking: the spread of cancer slowed significantly. This discovery not only answers a long-standing question but also identifies ALK7 as a promising new target for therapy.
Timing Is Everything
The researchers also uncovered another critical insight: the timing of treatment could make all the difference. Using the organ-on-chip platform, they explored whether ALK7 was most important at the beginning of the metastatic journey—when cancer cells first breach blood vessels—or later, when cells already circulating in the bloodstream attempt to colonize distant organs.
The findings were clear. Cancer cells without ALK7 were unable to enter blood vessels at all. But if researchers simulated a later stage, placing cancer cells inside the vessels, they spread rapidly regardless of whether ALK7 was blocked. This suggests a narrow but crucial therapeutic window.
“Once we miss this early opportunity to block ALK7 receptors, the cancer cells can freely circulate in the bloodstream and easily seed into other organs,” Lee said. “But if we can inhibit ALK7 at the cancer’s earliest and most vulnerable stage, we might see better outcomes for patients.”
A New Era of Cancer Research
Beyond its immediate implications for pancreatic cancer, this study highlights the power of organ-on-chip systems—miniaturized models that replicate the complexity of human tissues far more effectively than traditional animal studies. By recreating the tumor microenvironment in controlled laboratory settings, researchers can test how cancer cells interact with blood vessels, immune cells, and drugs in ways that were previously impossible.
This innovation opens doors far beyond pancreatic cancer. Other tumor types, each with unique microenvironments, could be studied in similar ways. Some cancers may rely on different molecular tricks to spread, while others might share ALK7’s reliance. Organ-on-chip systems could also be applied to study how immune cells infiltrate tumors or how therapies might be fine-tuned to block the earliest stages of metastasis.
“Some cancers have very different microenvironments so, potentially, ALK7 might show different impacts,” Lee noted. “I hope this study really opens a new avenue for cancer research.”
The Human Stakes
Behind every molecular pathway and experimental breakthrough lies a deeply human story. For patients and families facing pancreatic cancer, time is the most precious commodity, and current treatments too often fall short. Discoveries like this one shine a ray of hope in an otherwise grim landscape. By identifying a specific target like ALK7, scientists inch closer to therapies that could prevent metastasis before it begins—the critical step that turns a localized tumor into a life-threatening disease.
While much work remains—clinical trials, drug development, and safety testing—the study marks an important milestone. It transforms an age-old mystery into a concrete strategy: stop the cancer at the gates of the bloodstream, before it can invade the body’s most vital organs.
A Future with New Possibilities
The fight against pancreatic cancer has always seemed like an uphill battle. Yet every discovery brings us closer to shifting the odds. By revealing the hidden role of ALK7 and the narrow window when it can be stopped, Cornell’s research offers not only scientific insight but also a message of resilience. The disease may be aggressive, but so too is human ingenuity.
In the grand struggle between medicine and cancer, progress often comes in small, painstaking steps. But sometimes, a single breakthrough changes the way the field thinks about the problem entirely. This study may be one of those moments—an inflection point in our understanding of how one of the deadliest cancers spreads, and how, perhaps, it might one day be stopped.
More information: Anna M. Kolarzyk et al, Non-canonical ALK7 pathways promote pancreatic cancer metastasis through β-catenin/MMP-mediated basement membrane breakdown and intravasation, Molecular Cancer (2025). DOI: 10.1186/s12943-025-02384-w